Chemical engineering is an engineering field which deals with the study of the operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials into useful products. Chemical engineering uses principles of chemistry, physics, mathematics, biology, and economics to efficiently use, produce, design, transport and transform energy and materials. The work of chemical engineers can range from the utilization of nanotechnology and nanomaterials in the laboratory to large-scale industrial processes that convert chemicals, raw materials, living cells, microorganisms, and energy into useful forms and products. Chemical engineers are involved in many aspects of plant design and operation, including safety and hazard assessments, process design and analysis, modeling, control engineering, chemical reaction engineering, nuclear engineering, biological engineering, construction specification, and operating instructions.

Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still.

The chemical industry comprises the companies and other organizations that develop and produce industrial, specialty and other chemicals. Central to the modern world economy, it converts raw materials into commodity chemicals for industrial and consumer products. It includes industries for petrochemicals such as polymers for plastics and synthetic fibers; inorganic chemicals such as acids and alkalis; agricultural chemicals such as fertilizers, pesticides and herbicides; and other categories such as industrial gases, speciality chemicals and pharmaceuticals.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

An oil refinery or petroleum refinery is an industrial process plant where petroleum is transformed and refined into products such as gasoline (petrol), diesel fuel, asphalt base, fuel oils, heating oil, kerosene, liquefied petroleum gas and petroleum naphtha. Petrochemical feedstock like ethylene and propylene can also be produced directly by cracking crude oil without the need of using refined products of crude oil such as naphtha. The crude oil feedstock has typically been processed by an oil production plant. There is usually an oil depot at or near an oil refinery for the storage of incoming crude oil feedstock as well as bulk liquid products. In 2020, the total capacity of global refineries for crude oil was about 101.2 million barrels per day.
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation to fractionate. Generally the component parts have boiling points that differ by less than 25 °C (45 °F) from each other under a pressure of one atmosphere. If the difference in boiling points is greater than 25 °C, a simple distillation is typically used.

A refinery is a production facility composed of a group of chemical engineering unit processes and unit operations refining certain materials or converting raw material into products of value.

A fractionating column or fractional column is equipment used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on their differences in volatility. Fractionating columns are used in small-scale laboratory distillations as well as large-scale industrial distillations.
Process engineering is the understanding and application of the fundamental principles and laws of nature that allow humans to transform raw material and energy into products that are useful to society, at an industrial level. By taking advantage of the driving forces of nature such as pressure, temperature and concentration gradients, as well as the law of conservation of mass, process engineers can develop methods to synthesize and purify large quantities of desired chemical products. Process engineering focuses on the design, operation, control, optimization and intensification of chemical, physical, and biological processes. Process engineering encompasses a vast range of industries, such as agriculture, automotive, biotechnical, chemical, food, material development, mining, nuclear, petrochemical, pharmaceutical, and software development. The application of systematic computer-based methods to process engineering is "process systems engineering".

Industrial processes are procedures involving chemical, physical, electrical, or mechanical steps to aid in the manufacturing of an item or items, usually carried out on a very large scale. Industrial processes are the key components of heavy industry.

In chemical engineering and related fields, a unit operation is a basic step in a process. Unit operations involve a physical change or chemical transformation such as separation, crystallization, evaporation, filtration, polymerization, isomerization, and other reactions. For example, in milk processing, the following unit operations are involved: homogenization, pasteurization, and packaging. These unit operations are connected to create the overall process. A process may require many unit operations to obtain the desired product from the starting materials, or feedstocks.

A chemical plant is an industrial process plant that manufactures chemicals, usually on a large scale. The general objective of a chemical plant is to create new material wealth via the chemical or biological transformation and or separation of materials. Chemical plants use specialized equipment, units, and technology in the manufacturing process. Other kinds of plants, such as polymer, pharmaceutical, food, and some beverage production facilities, power plants, oil refineries or other refineries, natural gas processing and biochemical plants, water and wastewater treatment, and pollution control equipment use many technologies that have similarities to chemical plant technology such as fluid systems and chemical reactor systems. Some would consider an oil refinery or a pharmaceutical or polymer manufacturer to be effectively a chemical plant.

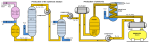
Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously fed into the process and separated fractions are removed continuously as output streams. Distillation is the separation or partial separation of a liquid feed mixture into components or fractions by selective boiling and condensation. The process produces at least two output fractions. These fractions include at least one volatile distillate fraction, which has boiled and been separately captured as a vapor condensed to a liquid, and practically always a bottoms fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.

A pilot plant is a pre-commercial production system that employs new production technology and/or produces small volumes of new technology-based products, mainly for the purpose of learning about the new technology. The knowledge obtained is then used for design of full-scale production systems and commercial products, as well as for identification of further research objectives and support of investment decisions. Other (non-technical) purposes include gaining public support for new technologies and questioning government regulations. Pilot plant is a relative term in the sense that pilot plants are typically smaller than full-scale production plants, but are built in a range of sizes. Also, as pilot plants are intended for learning, they typically are more flexible, possibly at the expense of economy. Some pilot plants are built in laboratories using stock lab equipment, while others require substantial engineering efforts, cost millions of dollars, and are custom-assembled and fabricated from process equipment, instrumentation and piping. They can also be used to train personnel for a full-scale plant. Pilot plants tend to be smaller compared to demonstration plants.
In chemical engineering, process design is the choice and sequencing of units for desired physical and/or chemical transformation of materials. Process design is central to chemical engineering, and it can be considered to be the summit of that field, bringing together all of the field's components.
The McCabe–Thiele method is a technique that is commonly employed in the field of chemical engineering to model the separation of two substances by a distillation column. It uses the fact that the composition at each theoretical tray is completely determined by the mole fraction of one of the two components. This method is based on the assumptions that the distillation column is isobaric—i.e the pressure remains constant—and that the flow rates of liquid and vapor do not change throughout the column. The assumption of constant molar overflow requires that:
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as an equilibrium stage, ideal stage, or a theoretical tray. The performance of many separation processes depends on having series of equilibrium stages and is enhanced by providing more such stages. In other words, having more theoretical plates increases the efficiency of the separation process be it either a distillation, absorption, chromatographic, adsorption or similar process.
The following outline is provided as an overview of and topical guide to chemical engineering:
A separation process is a method that converts a mixture or a solution of chemical substances into two or more distinct product mixtures, a scientific process of separating two or more substances in order to obtain purity. At least one product mixture from the separation is enriched in one or more of the source mixture's constituents. In some cases, a separation may fully divide the mixture into pure constituents. Separations exploit differences in chemical properties or physical properties between the constituents of a mixture.
Aspen Plus, Aspen HYSYS, ChemCad and MATLAB, PRO are the commonly used process simulators for modeling, simulation and optimization of a distillation process in the chemical industries. Distillation is the technique of preferential separation of the more volatile components from the less volatile ones in a feed followed by condensation. The vapor produced is richer in the more volatile components. The distribution of the component in the two phase is governed by the vapour-liquid equilibrium relationship. In practice, distillation may be carried out by either two principal methods. The first method is based on the production of vapor boiling the liquid mixture to be separated and condensing the vapors without allowing any liquid to return to the still. There is no reflux. The second method is based on the return of part of the condensate to still under such conditions that this returning liquid is brought into intimate contact with the vapors on their way to condenser.