Related Research Articles

George Charles de Hevesy was a Hungarian radiochemist and Nobel Prize in Chemistry laureate, recognized in 1943 for his key role in the development of radioactive tracers to study chemical processes such as in the metabolism of animals. He also co-discovered the element hafnium.

Aarhus University is a public research university with its main campus located in Aarhus, Denmark. It is the second largest and second oldest university in Denmark. The university is part of the Coimbra Group, the Guild, and Utrecht Network of European universities and is a member of the European University Association.

Jens Christian Skou was a Danish biochemist and Nobel laureate.

Raymond Urgel Lemieux, CC, AOE, FRS was a Canadian organic chemist, who pioneered many discoveries in the field of chemistry, his first and most famous being the synthesis of sucrose. His contributions include the discovery of the anomeric effect and the development of general methodologies for the synthesis of saccharides still employed in the area of carbohydrate chemistry. He was a fellow of the Royal Society of Canada and the Royal Society (England), and a recipient of the prestigious Albert Einstein World Award of Science and Wolf Prize in Chemistry.

The Baeyer–Villiger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant. The reaction is named after Adolf von Baeyer and Victor Villiger who first reported the reaction in 1899.

In organic chemistry, the anomeric effect or Edward-Lemieux effect is a stereoelectronic effect that describes the tendency of heteroatomic substituents adjacent to a heteroatom within a cyclohexane ring to prefer the axial orientation instead of the less hindered equatorial orientation that would be expected from steric considerations. This effect was originally observed in pyranose rings by J. T. Edward in 1955 when studying carbohydrate chemistry.

Ronald T. Raines is an American chemical biologist. He is the Roger and Georges Firmenich Professor of Natural Products Chemistry at the Massachusetts Institute of Technology. He is known for using ideas and methods of physical organic chemistry to solve important problems in biology.

Sir Alan Rushton Battersby was an English organic chemist best known for his work to define the chemical intermediates in the biosynthetic pathway to vitamin B12 and the reaction mechanisms of the enzymes involved. His research group was also notable for its synthesis of radiolabelled precursors to study alkaloid biosynthesis and the stereochemistry of enzymic reactions. He won numerous awards including the Royal Medal in 1984 and the Copley Medal in 2000. He was knighted in the 1992 New Year Honours. Battersby died in February 2018 at the age of 92.
Klaus Bechgaard was a Danish scientist and chemist, noted for being one of the first scientists in the world to synthesize a number of organic charge transfer complexes and demonstrate their superconductivity, therefore the name Bechgaard salt. These salts all exhibit superconductivity at low temperatures.
Morten Peter Meldal is a Danish chemist and Nobel laureate. He is a professor of chemistry at the University of Copenhagen in Copenhagen, Denmark. He is best known for developing the CuAAC-click reaction, concurrently with but independent of Valery V. Fokin and K. Barry Sharpless.
Carbohydrate conformation refers to the overall three-dimensional structure adopted by a carbohydrate (saccharide) molecule as a result of the through-bond and through-space physical forces it experiences arising from its molecular structure. The physical forces that dictate the three-dimensional shapes of all molecules—here, of all monosaccharide, oligosaccharide, and polysaccharide molecules—are sometimes summarily captured by such terms as "steric interactions" and "stereoelectronic effects".
A glycosyl donor is a carbohydrate mono- or oligosaccharide that will react with a suitable glycosyl acceptor to form a new glycosidic bond. By convention, the donor is the member of this pair that contains the resulting anomeric carbon of the new glycosidic bond. The resulting reaction is referred to as a glycosylation or chemical glycosylation.

An oxocarbeniumion is a chemical species characterized by a central sp2-hybridized carbon, an oxygen substituent, and an overall positive charge that is delocalized between the central carbon and oxygen atoms. An oxocarbenium ion is represented by two limiting resonance structures, one in the form of a carbenium ion with the positive charge on carbon and the other in the form of an oxonium species with the formal charge on oxygen. As a resonance hybrid, the true structure falls between the two. Compared to neutral carbonyl compounds like ketones or esters, the carbenium ion form is a larger contributor to the structure. They are common reactive intermediates in the hydrolysis of glycosidic bonds, and are a commonly used strategy for chemical glycosylation. These ions have since been proposed as reactive intermediates in a wide range of chemical transformations, and have been utilized in the total synthesis of several natural products. In addition, they commonly appear in mechanisms of enzyme-catalyzed biosynthesis and hydrolysis of carbohydrates in nature. Anthocyanins are natural flavylium dyes, which are stabilized oxocarbenium compounds. Anthocyanins are responsible for the colors of a wide variety of common flowers such as pansies and edible plants such as eggplant and blueberry.
The α-ketol rearrangement is the acid-, base-, or heat-induced 1,2-migration of an alkyl or aryl group in an α-hydroxy ketone or aldehyde to give an isomeric product.

Eluvathingal Devassy Jemmis or E. D. Jemmis is a professor of theoretical chemistry at the Indian Institute of Science, Bangalore, India. He was the founding director of Indian Institute of Science Education and Research, Thiruvananthapuram (IISER-TVM). His primary area of research is applied theoretical chemistry with emphasis on structure, bonding and reactivity, across the periodic table of the elements. Apart from many of his contributions to applied theoretical chemistry, an equivalent of the structural chemistry of carbon, as exemplified by the Huckel 4n+2 Rule, benzenoid aromatics and graphite, and tetrahedral carbon and diamond, is brought in the structural chemistry of boron by the Jemmis mno rules which relates polyhedral and macropolyhedral boranes to allotropes of boron and boron-rich solids. He has been awarded Padma Shri in Science and Engineering category by the Government of India.
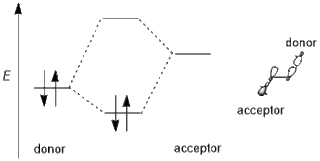
In chemistry, primarily organic and computational chemistry, a stereoelectronic effect is an effect on molecular geometry, reactivity, or physical properties due to spatial relationships in the molecules' electronic structure, in particular the interaction between atomic and/or molecular orbitals. Phrased differently, stereoelectronic effects can also be defined as the geometric constraints placed on the ground and/or transition states of molecules that arise from considerations of orbital overlap. Thus, a stereoelectronic effect explains a particular molecular property or reactivity by invoking stabilizing or destabilizing interactions that depend on the relative orientations of electrons in space.
Sabine Flitsch is a German organic chemist and chemical biologist who holds a personal chair in Chemical Biology at the University of Manchester School of Chemistry, where she runs an active research glycobiology research group based in the Manchester Interdisciplinary Biocentre.
Ove Christiansen is professor of chemistry at the Department of Chemistry, Aarhus University (AU), Denmark. He is contributor to the DALTON program package and initiated the MidasCpp program for the accurate description of nuclear dynamics with means of Coupled Cluster Theory.

Ashraf Brik is a full professor at the Schulich Faculty of Chemistry at the Technion Institute of Technology, Israel. His laboratory specializes in developing synthetic methods for chemical synthesis of proteins with post-translational modifications (PTMs) in quantities that allow for them to be studied thoroughly. Brik's group is developing novel modulators based on small molecules, peptides and peptidomimetics, to influence enzymes and proteins involved in various diseases. With the help of these tools, it is possible to understand how molecules and biological systems affect health.
Nicholas John Turner, is a British chemist and a Professor in the Department of Chemistry at The University of Manchester. His research in general is based on biochemistry and organic chemistry, specifically on biotechnology, cell biology, biocatalysis and organic synthesis.
References
- ↑ "Alle". 27 August 2007.
- ↑ Bols, M “1-Azasugars, Apparent Transition State Analogs of Equatorial Glycoside Formation/cleavage.” Acc. Chem. Res. 1998 31 1-8.
- ↑ Jensen, H. H.; Bols, M. ”Stereoelectronic Substituent Effects.” Acc. Chem. Res. 2006, 39, 259-265.
- ↑ Marinescu, L. G.; Bols, M. “Very High Rate Acceleration of Benzyl Alcohol Oxidation by an Artificial Enzyme.” Ang. Chem. Int. Ed. 2006 45 4590-4597.
- ↑ Bols, M. "Carbohydrate Building Blocks." John Wiley Science Publishers, New York 1996