Related Research Articles

In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present.
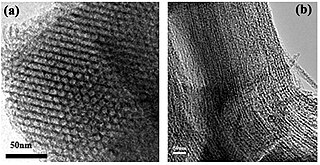
A mesoporous material is a nanoporous material containing pores with diameters between 2 and 50 nm, according to IUPAC nomenclature. For comparison, IUPAC defines microporous material as a material having pores smaller than 2 nm in diameter and macroporous material as a material having pores larger than 50 nm in diameter.

DNA replication licensing factor MCM6 is a protein that in humans is encoded by the MCM6 gene. MCM6 is one of the highly conserved mini-chromosome maintenance proteins (MCM) that are essential for the initiation of eukaryotic genome replication.

Cetrimonium bromide ([(C16H33)N(CH3)3]Br; cetyltrimethylammonium bromide; hexadecyltrimethylammonium bromide; CTAB) is a quaternary ammonium surfactant.

Dissolved organic carbon (DOC) is the fraction of organic carbon operationally defined as that which can pass through a filter with a pore size typically between 0.22 and 0.7 micrometers. The fraction remaining on the filter is called particulate organic carbon (POC).
Mesoporous silicates are silicates with a special morphology.

Mesoporous silica is a form of silica that is characterised by its mesoporous structure, that is, having pores that range from 2 nm to 50 nm in diameter. According to IUPAC's terminology, mesoporosity sits between microporous (<2 nm) and macroporous (>50 nm). Mesoporous silica is a relatively recent development in nanotechnology. The most common types of mesoporous nanoparticles are MCM-41 and SBA-15. Research continues on the particles, which have applications in catalysis, drug delivery and imaging. Mesoporous ordered silica films have been also obtained with different pore topologies.

DNA replication licensing factor MCM7 is a protein that in humans is encoded by the MCM7 gene.

DNA replication licensing factor MCM2 is a protein that in humans is encoded by the MCM2 gene.

DNA replication licensing factor MCM4 is a protein that in humans is encoded by the MCM4 gene.

Protein MCM10 homolog is a protein that in humans is encoded by the MCM10 gene. It is essential for activation of the Cdc45:Mcm2-7:GINS helicase, and thus required for proper DNA replication.

CDC45 is a protein that in humans is encoded by the CDC45L gene.

A silsesquioxane is an organosilicon compound with the chemical formula [RSiO3/2]n. Silsesquioxanes are colorless solids that adopt cage-like or polymeric structures with Si-O-Si linkages and tetrahedral Si vertices. Silsesquioxanes are members of polyoctahedral silsesquioxanes ("POSS"), which have attracted attention as preceramic polymer precursors to ceramic materials and nanocomposites. Diverse substituents (R) can be attached to the Si centers. The molecules are unusual because they feature an inorganic silicate core and an organic exterior. The silica core confers rigidity and thermal stability.

Polyethylenimine (PEI) or polyaziridine is a polymer with repeating units composed of the amine group and two carbon aliphatic CH2CH2 spacers. Linear polyethyleneimines contain all secondary amines, in contrast to branched PEIs which contain primary, secondary and tertiary amino groups. Totally branched, dendrimeric forms were also reported. PEI is produced on an industrial scale and finds many applications usually derived from its polycationic character.
Mesoporous organosilica are a type of silica containing organic groups that give rise to mesoporosity. They exhibit pore size ranging from 2 nm - 50 nm, depending on the organic substituents. In contrast, zeolites exhibit pore sizes less than a nanometer. PMOs have potential applications as catalysts, adsorbents, trapping agents, drug delivery agents, stationary phases in chromatography and chemical sensors.
Guillermo Carlos Bazan is an American chemist, material scientist, and academic.
Charles T. Kresge is a chemist and retired Chief Technology Officer (CTO) of Saudi Aramco. He was R&D Vice President at the Dow Chemical Company. His area of expertise is inorganic synthesis, and his primary field of research is in the area of crystalline aluminosilicate materials, particularly for the discovery of mesoporous molecular sieves.

MCM-41 is a mesoporous material with a hierarchical structure from a family of silicate and alumosilicate solids that were first developed by researchers at Mobil Oil Corporation and that can be used as catalysts or catalyst supports.
Ionosilicas are defined as organosilicas containing chemically bound ionic groups. They represent a class of mesoporous organosilicas.
Omar K. Farha is the Charles E. and Emma H. Morrison Professor in Chemistry at Northwestern University, an Executive Editor for ACS Applied Materials & Interfaces, and President of NuMat Technologies. His current research spans diverse areas of chemistry and materials science ranging from energy to defense-related challenges. Specifically, his research focuses on the rational design of metal-organic frameworks (MOFs) for applications sensing, catalysis, storage, separations, and water purification. His research accomplishments have been recognized by several awards and honors including a fellow of the European Academy of Sciences, a Fellow of the Academy of Arab Scientists, the Kuwait Prize, the Japanese Society of Coordination Chemistry “International award for creative work”, the Royal Society of Chemistry “Environment, Sustainability and Energy Division Early Career” Award, the American Chemical Society “The Satinder Ahuja Award for Young Investigators in Separation Science” and “ACS ENFL Emerging Researcher Award”, and an award established by the Department of Chemistry at Northwestern University in his honor: the Omar Farha Award for Research Leadership “awarded for stewardship, cooperation and leadership in the finest pursuit of research in chemistry” and given annually to an outstanding research scientist working in the department. Prof. Farha has been named a “Highly Cited Researcher” from 2014 to 2022. Prof. Farha is one of the Top 100 Chemists (#35) in the world (Research.com). Prof. Farha is the co-founder and president of NuMat Technologies, the first company to commercialized an engineered system-level product enabled by Metal-Organic Framework Materials.
References
- Kresge, C. T., Leonowicz, M. E., Roth, W. J., Vartuli, J. C. and Beck, J. S. Nature, 1992. 359: 710-712.
- Beck, J.S., Vartuli, J. C., Roth, W. J., Leonowicz, M. E., Kresge, C. T., Schmitt, K. D., Chu, C. T. D., Olson, D. H., Sheppard, E. W., McCullen, S. B., Higgins, J. B. and Schlenker, J. L. J. Am. Chem. Soc., 1992. 114: 10834-10843.
- Beck, J. S. U.S. Patent 5, 057, 296. 1991.
- Huo, Q., Margolese, D. I., Ciesla, U., Denuth, D. G., Feng, P., Gier, T. E., Sieger, P., Firouzi, A., Chmelka, B. F., Schuth, F. and Stucky, G. D. Chem. Mater.., 1994. 6: 1176-1191.
- Choma, J., Pikus, S. and Jaroniec, M. Appl. Surf. Sci., 2005. 252: 562-569.
- Hamoudi, S. and Belkacemi, K. J. Porous Mater., 2004. 11: 47-54.