
In molecular biology, a riboswitch is a regulatory segment of a messenger RNA molecule that binds a small molecule, resulting in a change in production of the proteins encoded by the mRNA. Thus, an mRNA that contains a riboswitch is directly involved in regulating its own activity, in response to the concentrations of its effector molecule. The discovery that modern organisms use RNA to bind small molecules, and discriminate against closely related analogs, expanded the known natural capabilities of RNA beyond its ability to code for proteins, catalyze reactions, or to bind other RNA or protein macromolecules.

Cobalamin riboswitch is a cis-regulatory element which is widely distributed in 5' untranslated regions of vitamin B12 (Cobalamin) related genes in bacteria.

The glucosamine-6-phosphate riboswitch ribozyme is an RNA structure that resides in the 5' untranslated region (UTR) of the mRNA transcript of the glmS gene. This RNA regulates the glmS gene by responding to concentrations of a specific metabolite, glucosamine-6-phosphate (GlcN6P), in addition to catalyzing a self-cleaving chemical reaction upon activation. This cleavage leads to the degradation of the mRNA that contains the ribozyme, and lowers production of GlcN6P. The glmS gene encodes for an enzyme glutamine-fructose-6-phosphate amidotransferase, which catalyzes the formation of GlcN6P, a compound essential for cell wall biosynthesis, from fructose-6-phosphate and glutamine. Thus, when GlcN6P levels are high, the glmS ribozyme is activated and the mRNA transcript is degraded but in the absence of GlcN6P the gene continues to be translated into glutamine-fructose-6-phosphate amidotransferase and GlcN6P is produced. GlcN6P is a cofactor for this cleavage reaction, as it directly participates as an acid-base catalyst. This RNA is the first riboswitch also found to be a self-cleaving ribozyme and, like many others, was discovered using a bioinformatics approach.

The SAM-II riboswitch is a RNA element found predominantly in alpha-proteobacteria that binds S-adenosyl methionine (SAM). Its structure and sequence appear to be unrelated to the SAM riboswitch found in Gram-positive bacteria. This SAM riboswitch is located upstream of the metA and metC genes in Agrobacterium tumefaciens, and other methionine and SAM biosynthesis genes in other alpha-proteobacteria. Like the other SAM riboswitch, it probably functions to turn off expression of these genes in response to elevated SAM levels. A significant variant of SAM-II riboswitches was found in Pelagibacter ubique and related marine bacteria and called SAM-V. Also, like many structured RNAs, SAM-II riboswitches can tolerate long loops between their stems.

The ykkC/yxkD leader is a conserved RNA structure found upstream of the ykkC and yxkD genes in Bacillus subtilis and related genes in other bacteria. The function of this family is unclear for many years although it has been suggested that it may function to switch on efflux pumps and detoxification systems in response to harmful environmental molecules. The Thermoanaerobacter tengcongensis sequence AE013027 overlaps with that of purine riboswitch suggesting that the two riboswitches may work in conjunction to regulate the upstream gene which codes for TTE0584 (Q8RC62), a member of the permease family.
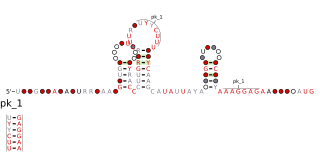
PreQ1-II riboswitches form a class of riboswitches that specifically bind pre-queuosine1 (PreQ1), a precursor of the modified nucleoside queuosine. They are found in certain species of Streptococcus and Lactococcus, and were originally identified as a conserved RNA secondary structure called the "COG4708 motif". All known members of this riboswitch class appear to control members of COG4708 genes. These genes are predicted to encode membrane-bound proteins and have been proposed to be a transporter of preQ1, or a related metabolite, based on their association with preQ1-binding riboswitches. PreQ1-II riboswitches have no apparent similarities in sequence or structure to preQ1-I riboswitches, a previously discovered class of preQ1-binding riboswitches. PreQ1 thus joins S-adenosylmethionine as the second metabolite to be found that is the ligand of more than one riboswitch class.
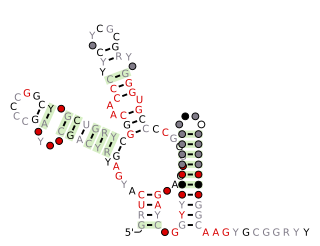
SAM-IV riboswitches are a kind of riboswitch that specifically binds S-adenosylmethionine (SAM), a cofactor used in many methylation reactions. Originally identified by bioinformatics, SAM-IV riboswitches are largely confined to the Actinomycetales, an order of Bacteria. Conserved features of SAM-IV riboswitch and experiments imply that they probably share a similar SAM-binding site to another class of SAM-binding riboswitches called SAM-I riboswitches. However, the scaffolds of these two types of riboswitch appear to be quite distinct.

SAH riboswitches are a kind of riboswitch that bind S-adenosylhomocysteine (SAH). When the coenzyme S-adenosylmethionine (SAM) is used in a methylation reaction, SAH is produced. SAH riboswitches typically up-regulate genes involved in recycling SAH to create more SAM. This is particularly relevant to cells, because high levels of SAH can be toxic. Originally identified by bioinformatics, SAH riboswitches are apparent in many species of bacteria, predominantly certain Proteobacteria and Actinobacteria. The atomic-resolution 3-dimensional structure of an SAH riboswitch has been solved using X-ray crystallography.

Cyclic di-GMP-I riboswitches are a class of riboswitch that specifically bind cyclic di-GMP, which is a second messenger that is used in a variety of microbial processes including virulence, motility and biofilm formation. Cyclic di-GMP-I riboswitches were originally identified by bioinformatics as a conserved RNA-like structure called the "GEMM motif". These riboswitches are present in a wide variety of bacteria, and are most common in Clostridia and certain varieties of Proteobacteria. The riboswitches are present in pathogens such as Clostridium difficile, Vibrio cholerae and Bacillus anthracis. Geobacter uraniumreducens is predicted to have 30 instances of this riboswitch in its genome. A bacteriophage that infects C. difficile is predicted to carry a cyclic di-GMP-I riboswitch, which it might use to detect and exploit the physiological state of bacteria that it infects.
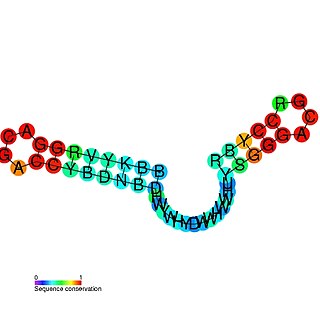
The mini-ykkC RNA motif was discovered as a putative RNA structure that is conserved in bacteria. The motif consists of two conserved stem-loops whose terminal loops contain the RNA sequence ACGR, where R represents either A or G. Mini-ykkC RNAs are widespread in Proteobacteria, but some are predicted in other phyla of bacteria. It was expected that the RNAs are cis-regulatory elements, because they are typically located upstream of protein-coding genes.

The sucA RNA motif is a conserved RNA structure found in bacteria of the order Burkholderiales. RNAs within this motif are always found in the presumed 5' UTR of sucA genes. sucA encodes a subunit of an enzyme that participates in the citric acid cycle by synthesizing succinyl-CoA from 2-oxoglutarate. A part of the conserved structure overlaps predicted Shine-Dalgarno sequences of the downstream sucA genes. Because of the RNA motif's consistent gene association and a possible mechanism for sequestering the ribosome binding site, it was proposed that the sucA RNA motif corresponds to a cis-regulatory element. Its relatively complex secondary structure could indicate that it is a riboswitch. However, the function of this RNA motif remains unknown.

The fluoride riboswitch is a conserved RNA structure identified by bioinformatics in a wide variety of bacteria and archaea. These RNAs were later shown to function as riboswitches that sense fluoride ions. These "fluoride riboswitches" increase expression of downstream genes when fluoride levels are elevated, and the genes are proposed to help mitigate the toxic effects of very high levels of fluoride.

The Downstream-peptide motif refers to a conserved RNA structure identified by bioinformatics in the cyanobacterial genera Synechococcus and Prochlorococcus and one phage that infects such bacteria. It was also detected in marine samples of DNA from uncultivated bacteria, which are presumably other species of cyanobacteria.

The glutamine riboswitch is a conserved RNA structure that was predicted by bioinformatics. It is present in a variety of lineages of cyanobacteria, as well as some phages that infect cyanobacteria. It is also found in DNA extracted from uncultivated bacteria living in the ocean that are presumably species of cyanobacteria.

The Moco-II RNA motif is a conserved RNA structure identified by bioinformatics. However, only 8 examples of the RNA motif are known. The RNAs are potentially in the 5' untranslated regions of genes related to molybdenum cofactor (Moco), specifically a gene that encodes a molybdenum-binding domain and a nitrate reductase, which uses Moco as a cofactor. Thus the RNA might be involved in the regulation of genes based on Moco levels. Reliable predictions of Moco-II RNAs are restricted to deltaproteobacteria, but a Moco-II RNA might be present in a betaproteobacterial species. The Moco RNA motif is another RNA that is associated with Moco, and its complex secondary structure and genetic experiments have led to proposals that it is a riboswitch. However, the simpler structure of the Moco-II RNA motif is less typical of riboswitches. Moco-II RNAs are typically followed by a predicted rho-independent transcription terminator.

The pan RNA motif defines a conserved RNA structure that was identified using bioinformatics. pan motif RNAs are present in three phyla: Chloroflexi, Firmicutes and Proteobacteria, although within the latter phylum they are only known in deltaproteobacteria. A pan RNA is present in the Firmicute Bacillus subtilis, which is one of the most extensively studied bacteria.

The pfl RNA motif refers to a conserved RNA structure present in some bacteria and originally discovered using bioinformatics. pfl RNAs are consistently present in genomic locations that likely correspond to the 5' untranslated regions of protein-coding genes. This arrangement in bacteria is commonly associated with cis-regulatory elements. Moreover, they are in presumed 5' UTRs of multiple non-homologous genes, suggesting that they function only in these locations. Additional evidence of cis-regulatory function came from the observation that predicted rho-independent transcription terminators overlap pfl RNAs. This overlap suggests that the alternate secondary structures of pfl RNA and the transcription terminator stem-loops compete with each other, and this is a common mechanism for cis gene control in bacteria.

The yjdF RNA motif is a conserved RNA structure identified using bioinformatics. Most yjdF RNAs are located in bacteria classified within the phylum Firmicutes. A yjdF RNA is found in the presumed 5' untranslated region of the yjdF gene in Bacillus subtilis, and almost all yjdF RNAs are found in the 5' UTRs of homologs of this gene. The function of the yjdF gene is unknown, but the protein that it is predicted to encode is classified by the Pfam Database as DUF2992.
The nadA RNA motif is a conserved RNA structure that was discovered by bioinformatics. The nadA motif is found in Acidobacteria.

The uup RNA motif is a conserved RNA structure that was discovered by bioinformatics. uup motif RNAs are found in Firmicutes and Gammaproteobacteria.




















