
The domestic silk moth is an insect from the moth family Bombycidae. It is the closest relative of Bombyx mandarina, the wild silk moth. The silkworm is the larva or caterpillar of a silk moth. It is an economically important insect, being a primary producer of silk. A silkworm's preferred food are white mulberry leaves, though they may eat other mulberry species and even the osage orange. Domestic silk moths are entirely dependent on humans for reproduction, as a result of millennia of selective breeding. Wild silk moths are not as commercially viable in the production of silk.
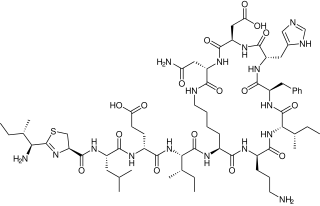
Nisin is a polycyclic antibacterial peptide produced by the bacterium Lactococcus lactis that is used as a food preservative. It has 34 amino acid residues, including the uncommon amino acids lanthionine (Lan), methyllanthionine (MeLan), didehydroalanine (Dha), and didehydroaminobutyric acid (Dhb). These unusual amino acids are introduced by posttranslational modification of the precursor peptide. In these reactions a ribosomally synthesized 57-mer is converted to the final peptide. The unsaturated amino acids originate from serine and threonine, and the enzyme-catalysed addition of cysteine residues to the didehydro amino acids result in the multiple (5) thioether bridges.

Polymyxins are antibiotics. Polymyxins B and E are used in the treatment of Gram-negative bacterial infections. They work mostly by breaking up the bacterial cell membrane. They are part of a broader class of molecules called nonribosomal peptides.
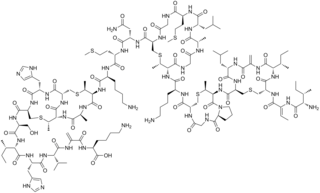
Bacitracin is a polypeptide antibiotic. It is a mixture of related cyclic peptides produced by Bacillus licheniformis bacteria, that was first isolated from the variety "Tracy I" in 1945. These peptides disrupt Gram-positive bacteria by interfering with cell wall and peptidoglycan synthesis.
Bombyx is the genus of true silk moths or mulberry silk moths of the family Bombycidae, also known as silkworms, which are the larvae or caterpillars of silk moths. The genus was erected as a subgenus by Carl Linnaeus in his 10th edition of Systema Naturae (1758).

The Silkworm is a 2014 crime fiction novel by J. K. Rowling, published under the pseudonym Robert Galbraith. It is the second novel in the Cormoran Strike series of detective novels and was followed by Career of Evil in 2015, Lethal White in 2018,Troubled Blood in 2020 and The Ink Black Heart in 2022.

Bombyx mandarina, the wild silk moth, is an insect from the moth family Bombycidae. It is the closest relative of Bombyx mori, the domesticated silk moth. The silkworm is the larva or caterpillar of a silk moth. Unlike the domesticated relative which is unable to fly or indeed persist outside human care, the wild silk moth is a fairly ordinary lepidopteran. Its main difference from the domesticated taxon is the more slender body with well-developed wings in males, and the dull greyish-brown colour.

Beta-defensin 2 (BD-2) also known as skin-antimicrobial peptide 1 (SAP1) is a peptide that in humans is encoded by the DEFB4 gene.

Alpha defensins are a family of mammalian defensin peptides of the alpha subfamily. In mammals they are also known as cryptdins and are produced within the small bowel. Cryptdin is a portmanteau of crypt and defensin.

Class II bacteriocins are a class of small peptides that inhibit the growth of various bacteria.

Arthropod defensins are a family defensin proteins found in mollusks, insects, and arachnids. These cysteine-rich antibacterial peptides are primarily active against Gram-positive bacteria and fungi in vitro. However Drosophila fruit flies mutant for the fly defensin were more susceptible to infection by the Gram-negative bacteria Providencia burhodogranariea, and resisted infection against Gram-positive bacteria like wild-type flies. It remains to be seen how in vitro activity relates to in vivo function. Mutants for the defensin-like antimicrobial peptide Drosomycin were more susceptible to fungi, validating a role for defensin-like peptides in anti-fungal defence.
Corazonin is a highly conserved neuropeptide found in many insects, in particular locusts and cockroaches.

Serratiopeptidase is a proteolytic enzyme (protease) produced by enterobacterium Serratia sp. E-15, now known as Serratia marcescens ATCC 21074. This microorganism was originally isolated in the late 1960s from silkworm intestine. Serratiopeptidase is present in the silkworm intestine and allows the emerging moth to dissolve its cocoon. Serratiopeptase is produced by purification from culture of Serratia E-15 bacteria. It is a member of the Peptidase M10B (Matrixin) family.

Wild silks have been known and used in many countries from early times, although the scale of production is far smaller than that from cultivated silkworms. Silk cocoons and nests often resemble paper or cloth, and their use has arisen independently in many societies.

Cecropins are antimicrobial peptides. They were first isolated from the hemolymph of Hyalophora cecropia, whence the term cecropin was derived. Cecropins lyse bacterial cell membranes; they also inhibit proline uptake and cause leaky membranes.
Gloverin is an inducible antibacterial insect protein which inhibits the synthesis of vital outer membrane proteins leading to a permeable outer membrane. Gloverin contains a large number of glycine residues.
Cephalosporins are a broad class of bactericidal antibiotics that include the β-lactam ring and share a structural similarity and mechanism of action with other β-lactam antibiotics. The cephalosporins have the ability to kill bacteria by inhibiting essential steps in the bacterial cell wall synthesis which in the end results in osmotic lysis and death of the bacterial cell. Cephalosporins are widely used antibiotics because of their clinical efficiency and desirable safety profile.
Myticin is a cysteine-rich peptide produced in three isoforms, A, B and C, by Mytilus galloprovincialis. Isoforms A and B show antibacterial activity against Gram-positive bacteria, while isoform C is additionally active against the fungus Fusarium oxysporum and bacterium Escherichia coli. Myticin-prepro is the precursor peptide.
Teixobactin is a peptide-like secondary metabolite of some species of bacteria, that kills some gram-positive bacteria. It appears to belong to a new class of antibiotics, and harms bacteria by binding to lipid II and lipid III, important precursor molecules for forming the cell wall.

Halovir refers to a multi-analogue compound belonging to a group of oligopeptides designated as lipopeptaibols which have membrane-modifying capacity and are fungal in origin. These peptides display interesting microheterogeneity; slight variation in encoding amino acids gives rise to a mixture of closely related analogues and have been shown to have antibacterial/antiviral properties.














