
Acetylcholine (ACh) is an organic chemical that functions in the brain and body of many types of animals as a neurotransmitter. Its name is derived from its chemical structure: it is an ester of acetic acid and choline. Parts in the body that use or are affected by acetylcholine are referred to as cholinergic. Substances that increase or decrease the overall activity of the cholinergic system are called cholinergics and anticholinergics, respectively.
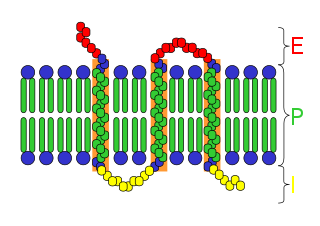
An integral, or intrinsic, membrane protein (IMP) is a type of membrane protein that is permanently attached to the biological membrane. All transmembrane proteins are IMPs, but not all IMPs are transmembrane proteins. IMPs comprise a significant fraction of the proteins encoded in an organism's genome. Proteins that cross the membrane are surrounded by annular lipids, which are defined as lipids that are in direct contact with a membrane protein. Such proteins can only be separated from the membranes by using detergents, nonpolar solvents, or sometimes denaturing agents.

Muscarine, L-(+)-muscarine, or muscarin is a natural product found in certain mushrooms, particularly in Inocybe and Clitocybe species, such as the deadly C. dealbata. Mushrooms in the genera Entoloma and Mycena have also been found to contain levels of muscarine which can be dangerous if ingested. Muscarine has been found in harmless trace amounts in Boletus, Hygrocybe, Lactarius and Russula. Trace concentrations of muscarine are also found in Amanita muscaria, though the pharmacologically more relevant compound from this mushroom is the Z-drug-like alkaloid muscimol. A. muscaria fruitbodies contain a variable dose of muscarine, usually around 0.0003% fresh weight. This is very low and toxicity symptoms occur very rarely. Inocybe and Clitocybe contain muscarine concentrations up to 1.6%.

Nicotinic acetylcholine receptors, or nAChRs, are receptor polypeptides that respond to the neurotransmitter acetylcholine. Nicotinic receptors also respond to drugs such as the agonist nicotine. They are found in the central and peripheral nervous system, muscle, and many other tissues of many organisms. At the neuromuscular junction they are the primary receptor in muscle for motor nerve-muscle communication that controls muscle contraction. In the peripheral nervous system: (1) they transmit outgoing signals from the presynaptic to the postsynaptic cells within the sympathetic and parasympathetic nervous system, and (2) they are the receptors found on skeletal muscle that receive acetylcholine released to signal for muscular contraction. In the immune system, nAChRs regulate inflammatory processes and signal through distinct intracellular pathways. In insects, the cholinergic system is limited to the central nervous system.

Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers in the parasympathetic nervous system.
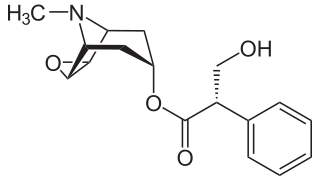
A muscarinic receptor antagonist (MRA) is a type of anticholinergic agent that blocks the activity of the muscarinic acetylcholine receptor. The muscarinic receptor is a protein involved in the transmission of signals through certain parts of the nervous system, and muscarinic receptor antagonists work to prevent this transmission from occurring. Notably, muscarinic antagonists reduce the activation of the parasympathetic nervous system. The normal function of the parasympathetic system is often summarised as "rest-and-digest", and includes slowing of the heart, an increased rate of digestion, narrowing of the airways, promotion of urination, and sexual arousal. Muscarinic antagonists counter this parasympathetic "rest-and-digest" response, and also work elsewhere in both the central and peripheral nervous systems.

The human muscarinic acetylcholine receptor M5, encoded by the CHRM5 gene, is a member of the G protein-coupled receptor superfamily of integral membrane proteins. It is coupled to Gq protein. Binding of the endogenous ligand acetylcholine to the M5 receptor triggers a number of cellular responses such as adenylate cyclase inhibition, phosphoinositide degradation, and potassium channel modulation. Muscarinic receptors mediate many of the effects of acetylcholine in the central and peripheral nervous system. The clinical implications of this receptor have not been fully explored; however, stimulation of this receptor is known to effectively decrease cyclic AMP levels and downregulate the activity of protein kinase A (PKA).

α-Cobratoxin is a substance of the venom of certain Naja cobras. It is a nicotinic acetylcholine receptor (nAChR) antagonist which causes paralysis by preventing the binding of acetylcholine to the nAChR.

The muscarinic acetylcholine receptor M1, also known as the cholinergic receptor, muscarinic 1, is a muscarinic receptor that in humans is encoded by the CHRM1 gene. It is localized to 11q13.

The muscarinic acetylcholine receptor M2, also known as the cholinergic receptor, muscarinic 2, is a muscarinic acetylcholine receptor that in humans is encoded by the CHRM2 gene. Multiple alternatively spliced transcript variants have been described for this gene.

The muscarinic acetylcholine receptor, also known as cholinergic/acetylcholine receptor M3, or the muscarinic 3, is a muscarinic acetylcholine receptor encoded by the human gene CHRM3.

The muscarinic acetylcholine receptor M4, also known as the cholinergic receptor, muscarinic 4 (CHRM4), is a protein that, in humans, is encoded by the CHRM4 gene.

Xanomeline is a small molecule muscarinic acetylcholine receptor agonist that was first synthesized in a collaboration between Eli Lilly and Novo Nordisk as an investigational therapeutic being studied for the treatment of central nervous system disorders.

Vedaclidine (INN, codenamed LY-297,802, NNC 11-1053) is an experimental analgesic drug which acts as a mixed agonist–antagonist at muscarinic acetylcholine receptors, being a potent and selective agonist for the M1 and M4 subtypes, yet an antagonist at the M2, M3 and M5 subtypes. It is orally active and an effective analgesic over 3× the potency of morphine, with side effects such as salivation and tremor only occurring at many times the effective analgesic dose. Human trials showed little potential for development of dependence or abuse, and research is continuing into possible clinical application in the treatment of neuropathic pain and cancer pain relief.

Muscarinic toxin 7 (MT7) is one member of a family of small peptides of 65 amino acid residues derived from the venom of African mamba snakes, which mainly target M1-subtype of muscarinic receptor. Muscarinic toxins like the nicotinic toxins have the three-finger fold structure, characteristic of the large superfamily of toxins that act at cholinergic synapses.

α-Neurotoxins are a group of neurotoxic peptides found in the venom of snakes in the families Elapidae and Hydrophiidae. They can cause paralysis, respiratory failure, and death. Members of the three-finger toxin protein family, they are antagonists of post-synaptic nicotinic acetylcholine receptors (nAChRs) in the neuromuscular synapse that bind competitively and irreversibly, preventing synaptic acetylcholine (ACh) from opening the ion channel. Over 100 α-neurotoxins have been identified and sequenced.

Muscarinic toxin 1 (MT1) belongs to the family of small peptides of 65 amino acid residues derived from the venom of African mamba snakes, with dual specificity for muscarinic receptor subtypes M1 and M4. Muscarinic toxins like the nicotinic toxins have the three-finger fold structure, characteristic of the large superfamily of toxins that act at cholinergic synapses.

κ-Bungarotoxin is a protein neurotoxin of the bungarotoxin family that is found in the venom of the many-banded krait, a snake found in Taiwan. κ-Bungarotoxin is a high affinity antagonist of nicotinic acetylcholine receptors (nAChRs), particularly of CHRNA3; it causes a post-synaptic blockade of neurotransmission. Although there is significant variability in the clinical effects of snake bites, neuromuscular paralysis and respiratory failure are associated with krait bites.
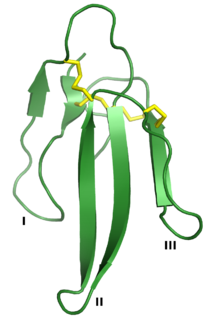
Three-finger toxins are a protein superfamily of small toxin proteins found in the venom of snakes. Three-finger toxins are in turn members of a larger superfamily of three-finger protein domains which includes non-toxic proteins that share a similar protein fold. The group is named for its common structure consisting of three beta strand loops connected to a central core containing four conserved disulfide bonds. The 3FP protein domain has no enzymatic activity and is typically between 60-74 amino acid residues long. Despite their conserved structure, three-finger toxin proteins have a wide range of pharmacological effects. Most members of the family are neurotoxins that act on cholinergic intercellular signaling; the alpha-neurotoxin family interacts with muscle nicotinic acetylcholine receptors (nAChRs), the kappa-bungarotoxin family with neuronal nAChRs, and muscarinic toxins with muscarinic acetylcholine receptors (mAChRs).

Three-finger proteins or three-finger protein domains are a protein superfamily consisting of small, roughly 60-80 amino acid residue protein domains with a common tertiary structure: three beta strand loops extended from a hydrophobic core stabilized by disulfide bonds. The family is named for the outstretched "fingers" of the three loops. Members of the family have no enzymatic activity, but are capable of forming protein-protein interactions with high specificity and affinity. The founding members of the family, also the best characterized by structure, are the three-finger toxins found in snake venom, which have a variety of pharmacological effects, most typically by disruption of cholinergic signaling. The family is also represented in non-toxic proteins, which have a wide taxonomic distribution; 3FP domains occur in the extracellular domains of some cell-surface receptors as well as in GPI-anchored and secreted globular proteins, usually involved in signaling.


















