Related Research Articles

In immunology, an antigen (Ag) is a molecule, moiety, foreign particulate matter, or an allergen, such as pollen, that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response.
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.

Telomerase, also called terminal transferase, is a ribonucleoprotein that adds a species-dependent telomere repeat sequence to the 3' end of telomeres. A telomere is a region of repetitive sequences at each end of the chromosomes of most eukaryotes. Telomeres protect the end of the chromosome from DNA damage or from fusion with neighbouring chromosomes. The fruit fly Drosophila melanogaster lacks telomerase, but instead uses retrotransposons to maintain telomeres.
A cancer vaccine, or oncovaccine, is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.

Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.
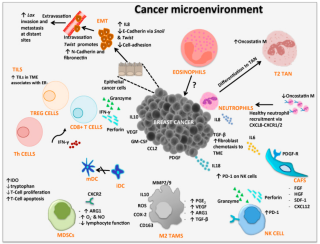
Cancer immunology (immuno-oncology) is an interdisciplinary branch of biology and a sub-discipline of immunology that is concerned with understanding the role of the immune system in the progression and development of cancer; the most well known application is cancer immunotherapy, which utilises the immune system as a treatment for cancer. Cancer immunosurveillance and immunoediting are based on protection against development of tumors in animal systems and (ii) identification of targets for immune recognition of human cancer.

Placenta-specific protein 1 is a small, secreted cell surface protein encoded on the X-chromosome by the PLAC1 gene. Since its discovery in 1999, PLAC1 has been found to play a role in placental development and maintenance, several gestational disorders including preeclampsia, fetal development and a large number of cancers.
Vaccine therapy is a type of treatment that uses a substance or group of substances to stimulate the immune system to destroy a tumor or infectious microorganisms such as bacteria or viruses.
Adoptive cell transfer (ACT) is the transfer of cells into a patient. The cells may have originated from the patient or from another individual. The cells are most commonly derived from the immune system with the goal of improving immune functionality and characteristics. In autologous cancer immunotherapy, T cells are extracted from the patient, genetically modified and cultured in vitro and returned to the same patient. Comparatively, allogeneic therapies involve cells isolated and expanded from a donor separate from the patient receiving the cells.
Gustav Gaudernack is a scientist working in the development of cancer vaccines and cancer immunotherapy. He has developed various strategies in immunological treatment of cancer. He is involved in several ongoing cellular and immuno-gene therapeutic clinical trials and his research group has put major efforts into the development of various T cell-based immunotherapeutic strategies.
Racotumomab is a therapeutic cancer vaccine for the treatment of solid tumors that is currently under clinical development by Recombio, an international public-private consortium with the participation of the Center of Molecular Immunology at Havana, Cuba (CIM) and researchers from Buenos Aires University and National University of Quilmes in Argentina. It induces the patient's immune system to generate a response against a cancer-specific molecular target with the purpose of blocking tumor growth, slowing disease progression and ultimately increasing patient survival.

Hans-Georg Rammensee is a German immunologist and cancer researcher. He has been Chair Professor and Head of the Department of Immunology at the University of Tübingen since 1996. Rammensee has contributed essentially to the research fields of MHC biology and tumor immunology and to the development of cancer immunotherapies.
Individualized medicine tailors treatment to a single patient. The term refers to an individual, truly personalized medicine that strives to treat each patient on the basis of his own individual biology.
Neoepitopes are a class of major histocompatibility complex (MHC) bounded peptides. They represent the antigenic determinants of neoantigens. Neoepitopes are recognized by the immune system as targets for T cells and can elicit immune response to cancer.
Individualized cancer immunotherapy, also referred to as individualized immuno-oncology, is a novel concept for therapeutic cancer vaccines that are truly personalized to a single individual.
RNA therapeutics are a new class of medications based on ribonucleic acid (RNA). Research has been working on clinical use since the 1990s, with significant success in cancer therapy in the early 2010s. In 2020 and 2021, mRNA vaccines have been developed globally for use in combating the coronavirus disease. The Pfizer–BioNTech COVID-19 vaccine was the first mRNA vaccine approved by a medicines regulator, followed by the Moderna COVID-19 vaccine, and others.

Uğur Şahin is a German oncologist and immunologist. He is the founder and CEO of BioNTech, which developed one of the major vaccines against COVID-19. His main fields of research are cancer research and immunology.

Özlem Türeci is a German physician, scientist and entrepreneur. In 2008, she co-founded the biotechnology company BioNTech, which in 2020 developed the first messenger RNA-based vaccine approved for use against COVID-19. Türeci has served as BioNTech's chief medical officer since 2018. Since 2021, she has been Professor of Personalized Immunotherapy at the Helmholtz Institute for Translational Oncology (HI-TRON) and Johannes Gutenberg University Mainz. Türeci and her spouse, Uğur Şahin, have won a number of awards.
Autogene cevumeran is an investigational mRNA vaccine being developed jointly by BioNTech and Genentech as an adjuvant therapy in cancer treatment to prevent cancer recurrence following surgery.
References
- 1 2 3 4 Vormehr, M; Diken, M; Boegel, S; Kreiter, S; Türeci, Ö; Sahin, U [in German] (2016). "Mutanome directed cancer immunotherapy". Current Opinion in Immunology. 39: 14–22. doi:10.1016/j.coi.2015.12.001. PMID 26716729.
- ↑ Stratton, MR; Campbell, PJ; Futreal, PA (2009). "The cancer genome". Nature. 458 (7239): 719–24. Bibcode:2009Natur.458..719S. doi:10.1038/nature07943. PMC 2821689 . PMID 19360079.
- ↑ Kreiter, S; Castle, JC; Türeci, Ö; Sahin, U [in German] (2012). "Targeting the tumor mutanome for personalized vaccination therapy". Oncoimmunology. 1 (5): 768–9. doi:10.4161/onci.19727. PMC 3429589 . PMID 22934277.
- ↑ Stratton, MR (2011). "Exploring the genomes of cancer cells: progress and promise". Science. 331 (6024): 1553–8. Bibcode:2011Sci...331.1553S. doi:10.1126/science.1204040. PMID 21436442. S2CID 7306827.
- 1 2 Overwijk, WW; Wang, E; Marincola, FM; Rammensee, HG; Restifo, NP (2013). "Mining the mutanome: developing highly personalized Immunotherapies based on mutational analysis of tumors". Journal for Immunotherapy of Cancer. 1: 11. doi: 10.1186/2051-1426-1-11 . PMC 4019909 . PMID 24829748.
- 1 2 Heemskerk, B; Kvistborg, P; Schumacher, TNM (2013). "The cancer antigenome". The EMBO Journal. 32 (2): 194–203. doi:10.1038/emboj.2012.333. PMC 3553384 . PMID 23258224.
- 1 2 3 4 Türeci, Ö; Vormehr, M; Diken, M; Kreiter, S; Huber, C; Sahin, U [in German] (2016). "Targeting the Heterogeneity of Cancer with Individualized Neoepitope Vaccines". Clinical Cancer Research. 22 (8): 1885–96. doi: 10.1158/1078-0432.CCR-15-1509 . PMID 27084742.
- 1 2 3 4 5 Castle, JC; Kreiter, S; Diekmann, J; Löwer, M; van de Roemer, N; de Graaf, J; Selmi, A; Diken, M; Boegel, S; Paret, C; Koslowski, M; Kuhn, AN; Britten, CM; Huber, C; Türeci, Ö; Sahin, U [in German] (2012). "Exploiting the mutanome for tumor vaccination". Cancer Research. 72 (5): 1081–91. doi: 10.1158/0008-5472.CAN-11-3722 . PMID 22237626.
- 1 2 Kreiter, S; Vormehr, M; van de Roemer, N; Diken, M; Löwer, M; Diekmann, J; Boegel, S; Schrörs, B; Vascotto, F; Castle, JC; Tadmor, AD; Schoenberger, SP; Huber, C; Türeci, Ö; Sahin, U [in German] (2015). "Mutant MHC class II epitopes drive therapeutic immune responses to cancer". Nature. 520 (7549): 692–6. Bibcode:2015Natur.520..692K. doi:10.1038/nature14426. PMC 4838069 . PMID 25901682.
- 1 2 3 Vormehr, M; Schrörs, B; Boegel, S; Löwer, M; Türeci, Ö; Sahin, U [in German] (2015). "Mutanome Engineered RNA Immunotherapy: Towards Patient-Centered Tumor Vaccination". Journal of Immunology Research. 2015: 595363. doi: 10.1155/2015/595363 . PMC 4710911 . PMID 26844233.
- 1 2 Matsushita, H; Vesely, MD; Koboldt, DC; Rickert, CG; Uppaluri, R; Magrini, VJ; Arthur, CD; White, JM; Chen, YS; Shea, LK; Hundal, J; Wendl, MC; Demeter, R; Wylie, T; Allison, JP; Smyth, MJ; Old, LJ; Mardis, ER; Schreiber, RD (2012). "Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting". Nature. 482 (7385): 400–4. Bibcode:2012Natur.482..400M. doi:10.1038/nature10755. PMC 3874809 . PMID 22318521.
- ↑ Katsnelson, A (2016). "Mutations as munitions: Neoantigen vaccines get a closer look as cancer treatment". Nature Medicine. 22 (2): 122–4. doi:10.1038/nm0216-122. PMID 26845402. S2CID 26454626.
- ↑ Vormehr, M; Türeci, Ö; Sahin, U [in German] (2019). "Harnessing Tumor Mutations for Truly Individualized Cancer Vaccines". Annual Review of Medicine. 70: 395–407. doi:10.1146/annurev-med-042617-101816. PMID 30691374. S2CID 59341051.
- 1 2 3 4 5 Sahin, U [in German]; Derhovanessian, E; Miller, M; Kloke, BP; Simon, P; Löwer, M; Bukur, V; Tadmor, AD; Luxemburger, U; Schrörs, B; Omokoko, T; Vormehr, M; Albrecht, C; Paruzynski, A; Kuhn, AN; Buck, J; Heesch, S; Schreeb, KH; Müller, F; Ortseifer, I; Vogler, I; Godehardt, E; Attig, S; Rae, R; Breitkreuz, A; Tolliver, C; Suchan, M; Martic, G; Hohberger, A; Sorn, P; Diekmann, J; Ciesla, J; Waksmann, O; Brück, AK; Witt, M; Zillgen, M; Rothermel, A; Kasemann, B; Langer, D; Bolte, S; Diken, M; Kreiter, S; Nemecek, R; Gebhardt, C; Grabbe, S; Höller, U; Utikal, J; Huber, C; Loquai, C; Türeci, Ö (2017). "Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer". Nature. 547 (7662): 222–6. Bibcode:2017Natur.547..222S. doi:10.1038/nature23003. PMID 28678784. S2CID 3757711.
- 1 2 Hilf, N; Kuttruff-Coqui, S; Frenzel, K; Bukur, V; Stevanović, S; Gouttefangeas, C; Platten, M; Tabatabai, G; Dutoit, V; van der Burg, SH; Thor Straten, P; Martínez-Ricarte, F; Ponsati, B; Okada, H; Lassen, U; Admon, A; Ottensmeier, CH; Ulges, A; Kreiter, S; von Deimling, A; Skardelly, M; Migliorini, D; Kroep, JR; Idorn, M; Rodon, J; Piró, J; Poulsen, HS; Shraibman, B; McCann, K; Mendrzyk, R; Löwer, M; Stieglbauer, M; Britten, CM; Capper, D; Welters, MJP; Sahuquillo, J; Kiesel, K; Derhovanessian, E; Rusch, E; Bunse, L; Song, C; Heesch, S; Wagner, C; Kemmer-Brück, A; Ludwig, J; Castle, JC; Schoor, O; Tadmor, AD; Green, E; Fritsche, J; Meyer, M; Pawlowski, N; Dorner, S; Hoffgaard, F; Rössler, M; Maurer, C; Weinschenk, T; Reinhardt, C; Huber, C; Rammensee, HG; Singh-Jasuja, H; Sahin, U [in German]; Dietrich, PY; Wick, W (2019). "Actively personalized vaccination trial for newly diagnosed glioblastoma". Nature. 565 (7738): 240–5. Bibcode:2019Natur.565..240H. doi:10.1038/s41586-018-0810-y. PMID 30568303. S2CID 56480674.
- 1 2 Kranz, LM; Diken, M; Haas, H; Kreiter, S; Loquai, C; Reuter, KC; Meng, M; Fritz, D; Vascotto, F; Hefesha, H; Grunwitz, C; Vormehr, M; Hüsemann, Y; Selmi, A; Kuhn, AN; Buck, J; Derhovanessian, E; Rae, R; Attig, S; Diekmann, J; Jabulowsky, RA; Heesch, S; Hassel, J; Langguth, P; Grabbe, S; Huber, C; Türeci, Ö; Sahin, U [in German] (2016). "Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy". Nature. 534 (7607): 396–401. Bibcode:2016Natur.534..396K. doi:10.1038/nature18300. PMID 27281205. S2CID 38112227.
- 1 2 Sahin, U [in German]; Karikó, K; Türeci, Ö (2014). "mRNA-based therapeutics--developing a new class of drugs". Nature Reviews Drug Discovery. 13 (10): 759–80. doi: 10.1038/nrd4278 . PMID 25233993. S2CID 27454546.