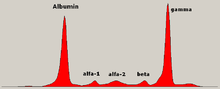
Serum protein electrophoresis is a laboratory test that examines specific proteins in the blood called globulins. The most common indications for a serum protein electrophoresis test are to diagnose or monitor multiple myeloma, a monoclonal gammopathy of uncertain significance (MGUS), or further investigate a discrepancy between a low albumin and a relatively high total protein. Unexplained bone pain, anemia, proteinuria, chronic kidney disease, and hypercalcemia are also signs of multiple myeloma, and indications for SPE. Blood must first be collected, usually into an airtight vial or syringe. Electrophoresis is a laboratory technique in which the blood serum is applied to either an acetate membrane soaked in a liquid buffer, or to a buffered agarose gel matrix, or into liquid in a capillary tube, and exposed to an electric current to separate the serum protein components into five major fractions by size and electrical charge: serum albumin, alpha-1 globulins, alpha-2 globulins, beta 1 and 2 globulins, and gamma globulins.
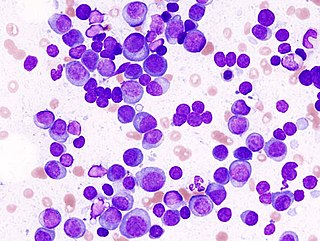
Multiple myeloma (MM), also known as plasma cell myeloma and simply myeloma, is a cancer of plasma cells, a type of white blood cell that normally produces antibodies. Often, no symptoms are noticed initially. As it progresses, bone pain, anemia, renal insufficiency, and infections may occur. Complications may include hypercalcemia and amyloidosis.
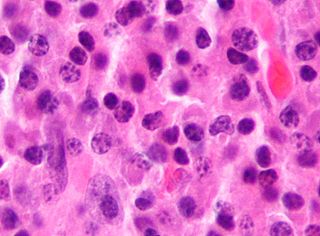
Plasma cells, also called plasma B cells or effector B cells, are white blood cells that originate in the lymphoid organs as B cells and secrete large quantities of proteins called antibodies in response to being presented specific substances called antigens. These antibodies are transported from the plasma cells by the blood plasma and the lymphatic system to the site of the target antigen, where they initiate its neutralization or destruction. B cells differentiate into plasma cells that produce antibody molecules closely modeled after the receptors of the precursor B cell.

POEMS syndrome is a rare paraneoplastic syndrome caused by a clone of aberrant plasma cells. The name POEMS is an acronym for some of the disease's major signs and symptoms, as is PEP.

Cryoglobulinemia is a medical condition in which the blood contains large amounts of pathological cold sensitive antibodies called cryoglobulins – proteins that become insoluble at reduced temperatures. This should be contrasted with cold agglutinins, which cause agglutination of red blood cells.

Monoclonal gammopathy of undetermined significance (MGUS) is a plasma cell dyscrasia in which plasma cells or other types of antibody-producing cells secrete a myeloma protein, i.e. an abnormal antibody, into the blood; this abnormal protein is usually found during standard laboratory blood or urine tests. MGUS resembles multiple myeloma and similar diseases, but the levels of antibodies are lower, the number of plasma cells in the bone marrow is lower, and it rarely has symptoms or major problems. However, since MGUS can lead to multiple myeloma, which develops at the rate of about 1.5% a year, or other symptomatic conditions, yearly monitoring is recommended.
Waldenström macroglobulinemia is a type of cancer affecting two types of B cells: lymphoplasmacytoid cells and plasma cells. Both cell types are white blood cells. It is characterized by having high levels of a circulating antibody, immunoglobulin M (IgM), which is made and secreted by the cells involved in the disease. Waldenström macroglobulinemia is an "indolent lymphoma" and a type of lymphoproliferative disease which shares clinical characteristics with the indolent non-Hodgkin lymphomas. It is commonly classified as a form of plasma cell dyscrasia, similar to other plasma cell dyscrasias that, for example, lead to multiple myeloma. Waldenström macroglobulinemia is commonly preceded by two clinically asymptomatic but progressively more pre-malignant phases, IgM monoclonal gammopathy of undetermined significance and smoldering Waldenström macroglobulinemia. The Waldenström macroglobulinemia spectrum of dysplasias differs from other spectrums of plasma cell dyscrasias in that it involves not only aberrant plasma cells but also aberrant lymphoplasmacytoid cells and that it involves IgM while other plasma dyscrasias involve other antibody isoforms.

Plasmacytoma is a plasma cell dyscrasia in which a plasma cell tumour grows within soft tissue or within the axial skeleton.

Monoclonal gammopathy, also known as paraproteinemia, is the presence of excessive amounts of myeloma protein or monoclonal gamma globulin in the blood. It is usually due to an underlying immunoproliferative disorder or hematologic neoplasms, especially multiple myeloma. It is sometimes considered equivalent to plasma cell dyscrasia. The most common form of the disease is monoclonal gammopathy of undetermined significance.

The immunoglobulin light chain is the small polypeptide subunit of an antibody (immunoglobulin).
In hematology, plasma cell dyscrasias are a spectrum of progressively more severe monoclonal gammopathies in which a clone or multiple clones of pre-malignant or malignant plasma cells over-produce and secrete into the blood stream a myeloma protein, i.e. an abnormal monoclonal antibody or portion thereof. The exception to this rule is the disorder termed non-secretory multiple myeloma; this disorder is a form of plasma cell dyscrasia in which no myeloma protein is detected in serum or urine of individuals who have clear evidence of an increase in clonal bone marrow plasma cells and/or evidence of clonal plasma cell-mediated tissue injury. Here, a clone of plasma cells refers to group of plasma cells that are abnormal in that they have an identical genetic identity and therefore are descendants of a single genetically distinct ancestor cell.

Plasma cell leukemia (PCL) is a plasma cell dyscrasia, i.e. a disease involving the malignant degeneration of a subtype of white blood cells called plasma cells. It is the terminal stage and most aggressive form of these dyscrasias, constituting 2% to 4% of all cases of plasma cell malignancies. PCL may present as primary plasma cell leukemia, i.e. in patients without prior history of a plasma cell dyscrasia or as secondary plasma cell dyscrasia, i.e. in patients previously diagnosed with a history of its predecessor dyscrasia, multiple myeloma. The two forms of PCL appear to be at least partially distinct from each other. In all cases, however, PCL is an extremely serious, life-threatening, and therapeutically challenging disease.
Free light chains (FLCs) are immunoglobulin light chains that are found in the serum (blood) in an unbound (free) state. In recent decades, measuring the amount of free light chains (FLCs) in the blood has become a practical clinical test. FLC tests can be used to diagnose and monitor diseases like multiple myeloma and amyloidosis.

Light chain deposition disease (LCDD) is a rare blood cell disease which is characterized by deposition of fragments of infection-fighting immunoglobulins, called light chains (LCs), in the body. LCs are normally cleared by the kidneys, but in LCDD, these light chain deposits damage organs and cause disease. The kidneys are almost always affected and this often leads to kidney failure. About half of people with light chain deposition disease also have a plasma cell dyscrasia, a spectrum of diseases that includes multiple myeloma, Waldenström's macroglobulinemia, and the monoclonal gammopathy of undetermined significance premalignant stages of these two diseases. Unlike in AL amyloidosis, in which light chains are laid down in characteristic amyloid deposits, in LCDD, light chains are deposited in non-amyloid granules.
Smouldering myeloma is a disease classified as intermediate in a spectrum of step-wise progressive diseases termed plasma cell dyscrasias. In this spectrum of diseases, a clone of plasma cells secreting monoclonal paraprotein causes the relatively benign disease of monoclonal gammopathy of undetermined significance. This clone proliferates and may slowly evolve into more aggressive sub-clones that cause smouldering multiple myeloma. Further and more rapid evolution causes the overtly malignant stage of multiple myeloma and can subsequently lead to the extremely malignant stage of secondary plasma cell leukemia. Thus, some patients with smouldering myeloma progress to multiple myeloma and plasma cell leukemia. Smouldering myeloma, however, is not a malignant disease. It is characterised as a pre-malignant disease that lacks symptoms but is associated with bone marrow biopsy showing the presence of an abnormal number of clonal myeloma cells, blood and/or urine containing a myeloma protein, and a significant risk of developing into a malignant disease.

Serum B-cell maturation antigen (sBCMA) is the cleaved form of B-cell maturation antigen (BCMA), found at low levels in the serum of normal patients and generally elevated in patients with multiple myeloma (MM). Changes in sBCMA levels have been found to correlate with a MM patient’s clinical status in response to treatment.
Monoclonal immunoglobulin deposition disease, or MIDD, is a disease characterised by the deposition of monoclonal immunoglobulins on the basement membrane of the kidney. Monoclonal immunoglobulins are produced by monoclonal plasma cells, which are found in a variety of plasma cell dyscrasias. The deposition of monoclonal immunoglobulins on the basement membrane of the kidney causes renal impairment. As well as the kidney, MIDD may also affect the liver, heart, peripheral nerves, lung and skin.
Crystal-storing histiocytosis is a form of histiocytosis that mostly occurs in people with monoclonal gammopathies. Histiocytosis is an excessive number of histiocytes. In the vast majority of crystal-storing histiocytosis cases, immunoglobulins accumulate within the cytoplasm of histiocytes; in rare cases clofazimine, cystine, silica, or Charcot–Leyden crystals may be found in the histiocytes instead. Non-immunoglobulin crystal-storing histiocytosis is mostly associated with non-malignant disorders, such as chronic inflammation or autoimmune abnormality conditions such as rheumatoid arthritis, Crohn's disease, or Helicobacter pylori gastritis. It may be a localised or generalised disease. Examples of locations where histiocytosis may occur include the lungs, pleura, stomach, kidney, bone marrow, thyroid, thymus, and parotid gland. The disease is described as generalised if two or more unrelated sites are involved.
Monoclonal gammopathy of renal significance (MGRS) are a group of kidney disorders that present with kidney damage due to nephrotoxic monoclonal immunoglobulins secreted by clonal plasma cells or B cells. By definition, people with MGRS do not meet criteria for multiple myeloma or other hematologic malignancies. The term MGRS was introduced in 2012 by the International Kidney and Monoclonal Gammopathy Research Group (IKMG). MGRS is associated with monoclonal gammopathy of undetermined significance (MGUS). People with MGUS have a monoclonal gammopathy but does not meet the criteria for the clonal burden nor the presence of end organ damage seen in hematologic malignancies. In a population based study based on the NHANES III health survey; 6% of patients with MGUS were subsequently classified as having MGRS. The prevalence and incidence of MGRS in the general population or in specific populations is not known but it is more prevalent in those over the age of 50 as there is a monoclonal protein (M-protein) present in 3% of those 50 and years older and 5% of those 70 years and older, placing those 50 and older at increased risk of MGRS.