
Harm reduction, or harm minimization, refers to a range of intentional practices and public health policies designed to lessen the negative social and/or physical consequences associated with various human behaviors, both legal and illegal. Harm reduction is used to decrease negative consequences of recreational drug use and sexual activity without requiring abstinence, recognizing that those unable or unwilling to stop can still make positive change to protect themselves and others.
The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the Department of Health and Social Care in the United Kingdom which is responsible for ensuring that medicines and medical devices work and are acceptably safe.
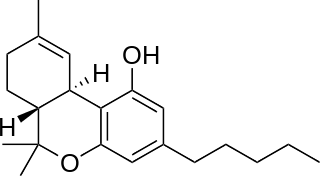
Nabiximols is a specific Cannabis extract that was approved in 2010 as a botanical drug in the United Kingdom. Nabiximols is sold as a mouth spray intended to alleviate neuropathic pain, spasticity, overactive bladder, and other symptoms of multiple sclerosis; it was developed by the UK company GW Pharmaceuticals. In 2019, it was proposed that following application of the spray, nabiximols is washed away from the oral mucosa by the saliva flow and ingested into the stomach, with subsequent absorption from the gastro-intestinal tract. Nabiximols is a combination drug standardized in composition, formulation, and dose. Its principal active components are the cannabinoids: tetrahydrocannabinol (THC) and cannabidiol (CBD). Each spray delivers a dose of 2.7 mg THC and 2.5 mg CBD.
Drug education is the planned provision of information, guidelines, resources, and skills relevant to living in a world where psychoactive substances are widely available and commonly used for a variety of both medical and non-medical purposes, some of which may lead to harms such as overdose, injury, infectious disease, or addiction.

The Alzheimer's Association was founded by Jerome H. Stone with the help of several family members in Chicago, Illinois. Incorporated on April 10, 1980, as the Alzheimer's Disease and Related Disorders Association, Inc., it is a non-profit American volunteer health organization which focuses on care, support and research for Alzheimer's disease.
Pharmaceutical policy is a branch of health policy that deals with the development, provision and use of medications within a health care system. It embraces drugs, biologics, vaccines and natural health products.

Lasofoxifene, sold under the brand name Fablyn, is a nonsteroidal selective estrogen receptor modulator (SERM) which is marketed by Pfizer in Lithuania and Portugal for the prevention and treatment of osteoporosis and for the treatment of vaginal atrophy, and the result of an exclusive research collaboration with Ligand Pharmaceuticals (LGND). It also appears to have had a statistically significant effect of reducing breast cancer in women according to a study published in The Journal of the National Cancer Institute.
Geoffrey William Guy is a British pharmacologist, physician, businessman and academic, who co-founded GW Pharmaceuticals and has developed treatments using compounds found in cannabis, which are the first cannabis-based medicines approved by and available on the British National Health Service (NHS).
The history of HIV/AIDS in Australia is distinctive, as Australian government bodies recognised and responded to the AIDS pandemic relatively swiftly, with the implementation of effective disease prevention and public health programs, such as needle and syringe programs (NSPs). As a result, despite significant numbers of at-risk group members contracting the virus in the early period following its discovery, Australia achieved and has maintained a low rate of HIV infection in comparison to the rest of the world.
Illicit drug use in Australia is the recreational use of prohibited drugs in Australia. Illicit drugs include illegal drugs, pharmaceutical drugs when used for non-medical purposes, and other substances used inappropriately. According to government and community organisations, the use and abuse, and the illegality, of illicit drugs is a social, health and legal issue that creates an annual illegal market estimated to be worth A$6.7 billion. Estimates made in 2022 place the figure at A$11.3 billion per year.

HIV/AIDS was first detected in Canada in 1982. In 2018, there were approximately 62,050 people living with HIV/AIDS in Canada. It was estimated that 8,300 people were living with undiagnosed HIV in 2018. Mortality has decreased due to medical advances against HIV/AIDS, especially highly active antiretroviral therapy (HAART).

Cannabis is a plant used in Australia for recreational, medicinal and industrial purposes. In 2019, 36% of Australians over the age of fourteen years had used cannabis in their lifetime and 11.6% had used cannabis in the last 12 months.
Michael Cowley FTSE is an Australian physiologist. He is best known for his mapping of the neural circuits involved in metabolism and obesity and diabetes treatment. He is a professor in the Department of Physiology at Monash University in the Faculty of Biomedical and Psychological Sciences. He is also a director of the Australian diabetes drug development company, Verva Inc, and director of the Monash Obesity & Diabetes Institute] (modi).

The Federal Ministry of Health is one of the Federal Ministries of Nigeria concerned with the formulation and implementation of policies related to health. It is headed by two Ministers appointed by the President, assisted by a Permanent Secretary, who is a career civil servant. The current Minister of Health is Muhammad Ali Pate. The current Minister of State for Health is Tunji Alausa. The Federal Ministry Of Health has over 800 workers consisting of closely 430 women and 370 men.[5]

The International Nonproprietary Name dronabinol, also known under the trade names Marinol, Syndros, Reduvo and Adversa, is a generic name for the molecule of delta-9-tetrahydrocannabinol in the pharmaceutical context. It has indications as an appetite stimulant, antiemetic, and sleep apnea reliever and is approved by the FDA as safe and effective for HIV/AIDS-induced anorexia and chemotherapy-induced nausea and vomiting only.

GW Pharmaceuticals Limited is a British pharmaceutics company known for its multiple sclerosis treatment product nabiximols which was the first natural cannabis plant derivative to gain market approval in any country. Another cannabis-based product, Epidiolex, was approved for treatment of epilepsy by the US Food and Drug Administration in 2018. It is a subsidiary of Jazz Pharmaceuticals.

Cannabis in Sweden is illegal for all purposes. It is illegal for recreational purposes, for most medical purposes and possession of even small amounts of cannabis is a criminal offence. Consequently, limited medical usage of cannabis-based drugs is only allowed for specific conditions.

The 21st Century Cures Act is a United States law enacted by the 114th United States Congress in December 2016 and then signed into law on December 13, 2016. It authorized $6.3 billion in funding, mostly for the National Institutes of Health. The act was supported especially by large pharmaceutical manufacturers and was opposed especially by some consumer organizations.











