
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and may require a mordant to improve the fastness of the dye on the fiber.
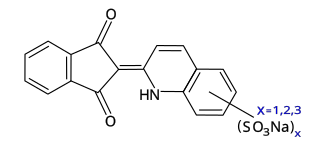
Quinoline Yellow WS is a mixture of organic compounds derived from the dye Quinoline Yellow SS. Owing to the presence of sulfonate groups, the WS dyes are water-soluble (WS). It is a mixture of disulfonates (principally), monosulfonates and trisulfonates of 2-(2-quinolyl)indan-1,3-dione with a maximum absorption wavelength of 416 nm.p. 119

Alizarin is an organic compound with formula C
14H
8O
4 that has been used throughout history as a prominent red dye, principally for dyeing textile fabrics. Historically it was derived from the roots of plants of the madder genus. In 1869, it became the first natural dye to be produced synthetically.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology, in cytology, and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.
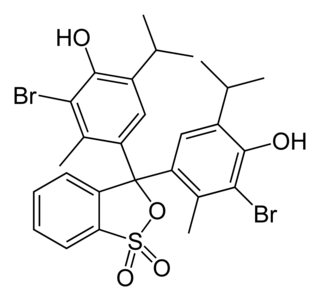
Bromothymol blue is a pH indicator. It is mostly used in applications that require measuring substances that would have a relatively neutral pH. A common use is for measuring the presence of carbonic acid in a liquid. It is typically sold in solid form as the sodium salt of the acid indicator.

Potassium dichromate, K2Cr2O7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color. The salt is popular in laboratories because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.
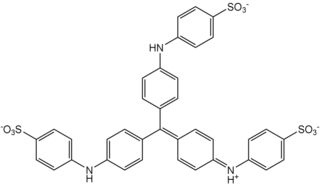
Methyl blue is a chemical compound with the molecular formula C37H27N3Na2O9S3. It is used as a stain in histology, and stains collagen blue in tissue sections. It can be used in some differential staining techniques such as Mallory's connective tissue stain and Gömöri trichrome stain, and can be used to mediate electron transfer in microbial fuel cells. Fungal cell walls are also stained by methyl blue.

Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet has antibacterial, antifungal, and anthelmintic (vermicide) properties and was formerly important as a topical antiseptic. The medical use of the dye has been largely superseded by more modern drugs, although it is still listed by the World Health Organization.

An acid dye is a dye that is typically applied to a textile at low pH. They are mainly used to dye wool, not cotton fabrics. Some acid dyes are used as food colorants, and some can also be used to stain organelles in the medical field.

Texas Red or sulforhodamine 101 acid chloride is a red fluorescent dye, used in histology for staining cell specimens, for sorting cells with fluorescent-activated cell sorting machines, in fluorescence microscopy applications, and in immunohistochemistry. Texas Red fluoresces at about 615 nm, and the peak of its absorption spectrum is at 589 nm. The powder is dark purple. Solutions can be excited by a dye laser tuned to 595-605 nm, or less efficiently a krypton laser at 567 nm. The absorption extinction coefficient at 596 nm is about 85,000 M−1cm−1.

Manganese carbonate is a compound with the chemical formula MnCO3. Manganese carbonate occurs naturally as the mineral rhodochrosite but it is typically produced industrially. It is a pale pink, water-insoluble solid. Approximately 20,000 metric tonnes were produced in 2005.

Ponceau S, Acid Red 112, or C.I. 27195 is a sodium salt of a diazo dye of a light red color, that may be used to prepare a stain for rapid reversible detection of protein bands on nitrocellulose or polyvinylidene fluoride (PVDF) membranes, as well as on cellulose acetate membranes. A Ponceau S stain is useful because it does not appear to have a deleterious effect on the sequencing of blotted polypeptides and is therefore one method of choice for locating polypeptides on western blots for blot-sequencing. It is also easily reversed with water washes, facilitating subsequent immunological detection. The stain can be completely removed from the protein bands by continued washing. Common stain formulations include 0.1% (w/v) Ponceau S in 5% acetic acid or 2% (w/v) Ponceau S in 30% trichloroacetic acid and 30% sulfosalicylic acid.

Aniline Blue WS, also called aniline blue, diphenylamine blue, China blue, or Soluble blue, is a mixture of methyl blue and water blue. It may also be either one of them. It is a soluble dye used as a biological dye, in fluorescence microscopy, appearing a yellow-green colour after excitation with violet light. It is a mixture of the trisulfonates of triphenyl rosaniline and of diphenyl rosaniline.

Water blue, also known as aniline blue, Acid blue 22, Soluble Blue 3M, Marine Blue V, or C.I. 42755, is a chemical compound used as a stain in histology. Water blue stains collagen blue in tissue sections. It is soluble in water and slightly soluble in ethanol.

Ponceau 2R, Xylidine ponceau, Ponceau G, Red R, Acid Red 26, Food Red 5, or C.I. 16150 is a red azo dye used in histology for staining. It is easily soluble in water and slightly in ethanol. It usually comes as a disodium salt.
Bismarck brown Y also called C.I. 21000 and C.I. Basic Brown 1, is a diazo dye with the idealized formula [(H2N)2C6H3N2]2C6H4. The dye is a mixture of closely related compounds. It was one of the earliest azo dyes, being described in 1863 by German chemist Carl Alexander von Martius. It is used in histology for staining tissues.

Hematoxylin and eosin stain is one of the principal tissue stains used in histology. It is the most widely used stain in medical diagnosis and is often the gold standard. For example, when a pathologist looks at a biopsy of a suspected cancer, the histological section is likely to be stained with H&E.
In microscopy, negative staining is an established method, often used in diagnostic microscopy, for contrasting a thin specimen with an optically opaque fluid. In this technique, the background is stained, leaving the actual specimen untouched, and thus visible. This contrasts with positive staining, in which the actual specimen is stained.
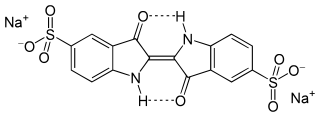
Indigo carmine, or 5,5′-indigodisulfonic acid sodium salt, is an organic salt derived from indigo by aromatic sulfonation, which renders the compound soluble in water. It is approved for use as a food colorant in the United States and European Union to produce a blue color. It has the E number E132, and is named Blue No. 2 by the Federal Food, Drug, and Cosmetic Act. It is also a pH indicator.

Alcian blue is any member of a family of polyvalent basic dyes, of which the Alcian blue 8G has been historically the most common and the most reliable member. It is used to stain acidic polysaccharides such as glycosaminoglycans in cartilages and other body structures, some types of mucopolysaccharides, sialylated glycocalyx of cells etc. For many of these targets it is one of the most widely used cationic dyes for both light and electron microscopy. Use of alcian blue has historically been a popular staining method in histology especially for light microscopy in paraffin embedded sections and in semithin resin sections. The tissue parts that specifically stain by this dye become blue to bluish-green after staining and are called "Alcianophilic". Alcian blue staining can be combined with H&E staining, PAS staining and van Gieson staining methods. Alcian blue can be used to quantitate acidic glycans both in microspectrophotometric quantitation in solution or for staining glycoproteins in polyacrylamide gels or on western blots. Biochemists had used it to assay acid polysaccharides in urine since the 1960s for diagnosis of diseases like mucopolysaccharidosis but from 1970's, partly due to lack of availability of Alcian and partly due to length and tediousness of the procedure, alternative methods had to be developed e.g. Dimethyl methylene blue method.

















