Related Research Articles

An axon or nerve fiber is a long, slender projection of a nerve cell, or neuron, in vertebrates, that typically conducts electrical impulses known as action potentials away from the nerve cell body. The function of the axon is to transmit information to different neurons, muscles, and glands. In certain sensory neurons, such as those for touch and warmth, the axons are called afferent nerve fibers and the electrical impulse travels along these from the periphery to the cell body and from the cell body to the spinal cord along another branch of the same axon. Axon dysfunction can be the cause of many inherited and acquired neurological disorders that affect both the peripheral and central neurons. Nerve fibers are classed into three types – group A nerve fibers, group B nerve fibers, and group C nerve fibers. Groups A and B are myelinated, and group C are unmyelinated. These groups include both sensory fibers and motor fibers. Another classification groups only the sensory fibers as Type I, Type II, Type III, and Type IV.

Myelin is a lipid-rich material that surrounds nerve cell axons to insulate them and increase the rate at which electrical impulses pass along the axon. The myelinated axon can be likened to an electrical wire with insulating material (myelin) around it. However, unlike the plastic covering on an electrical wire, myelin does not form a single long sheath over the entire length of the axon. Rather, myelin ensheaths the axon segmentally: in general, each axon is encased in multiple long sheaths with short gaps between, called nodes of Ranvier. At the nodes of Ranvier, which are approximately one thousandth of a mm in length, the axon's membrane is bare of myelin.
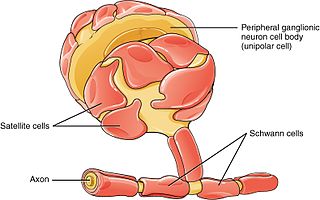
Schwann cells or neurolemmocytes are the principal glia of the peripheral nervous system (PNS). Glial cells function to support neurons and in the PNS, also include satellite cells, olfactory ensheathing cells, enteric glia and glia that reside at sensory nerve endings, such as the Pacinian corpuscle. The two types of Schwann cells are myelinating and nonmyelinating. Myelinating Schwann cells wrap around axons of motor and sensory neurons to form the myelin sheath. The Schwann cell promoter is present in the downstream region of the human dystrophin gene that gives shortened transcript that are again synthesized in a tissue-specific manner.
Nervous tissue, also called neural tissue, is the main tissue component of the nervous system. The nervous system regulates and controls body functions and activity. It consists of two parts: the central nervous system (CNS) comprising the brain and spinal cord, and the peripheral nervous system (PNS) comprising the branching peripheral nerves. It is composed of neurons, also known as nerve cells, which receive and transmit impulses, and neuroglia, also known as glial cells or glia, which assist the propagation of the nerve impulse as well as provide nutrients to the neurons.
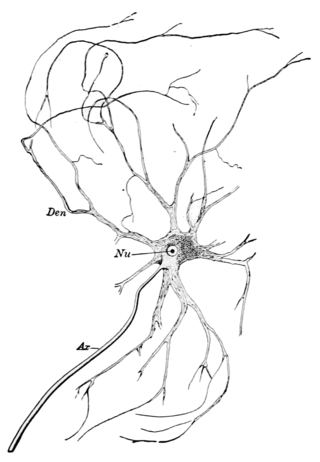
A motor nerve is a nerve that transmits motor signals from the central nervous system (CNS) to the muscles of the body. This is different from the motor neuron, which includes a cell body and branching of dendrites, while the nerve is made up of a bundle of axons. Motor nerves act as efferent nerves which carry information out from the CNS to muscles, as opposed to afferent nerves, which transfer signals from sensory receptors in the periphery to the CNS. Efferent nerves can also connect to glands or other organs/issues instead of muscles. The vast majority of nerves contain both sensory and motor fibers and are therefore called mixed nerves.

Glia, also called glial cells (gliocytes) or neuroglia, are non-neuronal cells in the central nervous system and the peripheral nervous system that do not produce electrical impulses. The neuroglia make up more than one half the volume of neural tissue in our body. They maintain homeostasis, form myelin in the peripheral nervous system, and provide support and protection for neurons. In the central nervous system, glial cells include oligodendrocytes, astrocytes, ependymal cells and microglia, and in the peripheral nervous system they include Schwann cells and satellite cells.
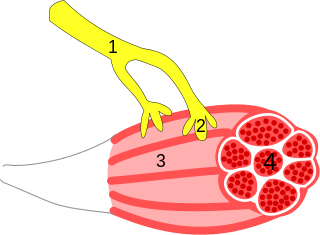
A neuroeffector junction is a site where a motor neuron releases a neurotransmitter to affect a target—non-neuronal—cell. This junction functions like a synapse. However, unlike most neurons, somatic efferent motor neurons innervate skeletal muscle, and are always excitatory. Visceral efferent neurons innervate smooth muscle, cardiac muscle, and glands, and have the ability to be either excitatory or inhibitory in function. Neuroeffector junctions are known as neuromuscular junctions when the target cell is a muscle fiber.
A neuromuscular junction is a chemical synapse between a motor neuron and a muscle fiber.

Tactile corpuscles or Meissner's corpuscles are a type of mechanoreceptor discovered by anatomist Georg Meissner (1829–1905) and Rudolf Wagner. This corpuscle is a type of nerve ending in the skin that is responsible for sensitivity to pressure. In particular, they have their highest sensitivity when sensing vibrations between 10 and 50 hertz. They are rapidly adaptive receptors. They are most concentrated in thick hairless skin, especially at the finger pads.
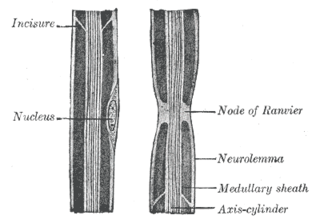
In neuroscience and anatomy, nodes of Ranvier, also known as myelin-sheath gaps, occur along a myelinated axon where the axolemma is exposed to the extracellular space. Nodes of Ranvier are uninsulated and highly enriched in ion channels, allowing them to participate in the exchange of ions required to regenerate the action potential. Nerve conduction in myelinated axons is referred to as saltatory conduction due to the manner in which the action potential seems to "jump" from one node to the next along the axon. This results in faster conduction of the action potential.

Wallerian degeneration is an active process of degeneration that results when a nerve fiber is cut or crushed and the part of the axon distal to the injury degenerates. A related process of dying back or retrograde degeneration known as 'Wallerian-like degeneration' occurs in many neurodegenerative diseases, especially those where axonal transport is impaired such as ALS and Alzheimer's disease. Primary culture studies suggest that a failure to deliver sufficient quantities of the essential axonal protein NMNAT2 is a key initiating event.
Synaptogenesis is the formation of synapses between neurons in the nervous system. Although it occurs throughout a healthy person's lifespan, an explosion of synapse formation occurs during early brain development, known as exuberant synaptogenesis. Synaptogenesis is particularly important during an individual's critical period, during which there is a certain degree of synaptic pruning due to competition for neural growth factors by neurons and synapses. Processes that are not used, or inhibited during their critical period will fail to develop normally later on in life.

The neuron doctrine is the concept that the nervous system is made up of discrete individual cells, a discovery due to decisive neuro-anatomical work of Santiago Ramón y Cajal and later presented by, among others, H. Waldeyer-Hartz. The term neuron was itself coined by Waldeyer as a way of identifying the cells in question. The neuron doctrine, as it became known, served to position neurons as special cases under the broader cell theory evolved some decades earlier. He appropriated the concept not from his own research but from the disparate observation of the histological work of Albert von Kölliker, Camillo Golgi, Franz Nissl, Santiago Ramón y Cajal, Auguste Forel and others.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.

Group C nerve fibers are one of three classes of nerve fiber in the central nervous system (CNS) and peripheral nervous system (PNS). The C group fibers are unmyelinated and have a small diameter and low conduction velocity, whereas Groups A and B are myelinated. Group C fibers include postganglionic fibers in the autonomic nervous system (ANS), and nerve fibers at the dorsal roots. These fibers carry sensory information.

Semaphorin-3A is a protein that in humans is encoded by the SEMA3A gene.

Axon terminals are distal terminations of the branches of an axon. An axon, also called a nerve fiber, is a long, slender projection of a nerve cell that conducts electrical impulses called action potentials away from the neuron's cell body in order to transmit those impulses to other neurons, muscle cells or glands. In the central nervous system, most presynaptic terminals are actually formed along the axons, not at their ends.
Perisynaptic schwann cells are neuroglia found at the Neuromuscular junction (NMJ) with known functions in synaptic transmission, synaptogenesis, and nerve regeneration. These cells share a common ancestor with both Myelinating and Non-Myelinating Schwann Cells called Neural Crest cells. Perisynaptic Schwann Cells (PSCs) contribute to the tripartite synapse organization in combination with the pre-synaptic nerve and the post-synaptic muscle fiber. PSCs are considered to be the glial component of the Neuromuscular Junction (NMJ) and have a similar functionality to that of Astrocytes in the Central Nervous System. The characteristics of PSCs are based on both external synaptic properties and internal glial properties, where the internal characteristics of PSCs develop based on the associated synapse, for example: the PSCs of a fast-twitch muscle fiber differ from the PSCs of a slow-twitch muscle fiber even when removed from their natural synaptic environment. PSCs of fast-twitch muscle fibers have higher Calcium levels in response to synapse innervation when compared to slow-twitch PSCs. This balance between external and internal influences creates a range of PSCs that are present in the many Neuromuscular Junctions of the Peripheral Nervous System.

Tripartite synapse refers to the functional integration and physical proximity of:
References
- 1 2 3 4 Griffin JW, Thompson WJ (November 2008). "Biology and pathology of nonmyelinating Schwann cells". Glia. 56 (14): 1518–1531. doi:10.1002/glia.20778. PMID 18803315.
- ↑ Harty BL, Monk KR (December 2017). "Unwrapping the unappreciated: recent progress in Remak Schwann cell biology". Current Opinion in Neurobiology. 47: 131–137. doi:10.1016/j.conb.2017.10.003. PMC 5963510 . PMID 29096241.
- ↑ Feng Z, Ko CP (September 2008). "Schwann cells promote synaptogenesis at the neuromuscular junction via transforming growth factor-beta1". The Journal of Neuroscience. 28 (39): 9599–9609. doi:10.1523/JNEUROSCI.2589-08.2008. PMC 3844879 . PMID 18815246.