Related Research Articles
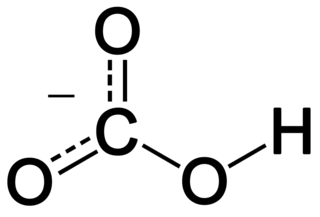
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula HCO−
3.

Carbon dioxide is an acidic colorless gas with a density about 53% higher than that of dry air. Carbon dioxide molecules consist of a carbon atom covalently double bonded to two oxygen atoms. It occurs naturally in Earth's atmosphere as a trace gas. The current concentration is about 0.04% (412 ppm) by volume, having risen from pre-industrial levels of 280 ppm. Natural sources include volcanoes, hot springs and geysers, and it is freed from carbonate rocks by dissolution in water and acids. Because carbon dioxide is soluble in water, it occurs naturally in groundwater, rivers and lakes, ice caps, glaciers and seawater. It is present in deposits of petroleum and natural gas. Carbon dioxide has a sharp and acidic odor and generates the taste of soda water in the mouth. However, at normally encountered concentrations it is odorless.
The Eocene Epoch is a geological epoch that lasted from about 56 to 33.9 million years ago (mya). It is the second epoch of the Paleogene Period in the modern Cenozoic Era. The name Eocene comes from the Ancient Greek ἠώς and καινός and refers to the "dawn" of modern ('new') fauna that appeared during the epoch.

Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's metabolic activities. This chemical energy is stored in carbohydrate molecules, such as sugars and starches, which are synthesized from carbon dioxide and water – hence the name photosynthesis, from the Greek phōs (φῶς), "light", and sunthesis (σύνθεσις), "putting together". In most cases, oxygen is also released as a waste product. Most plants, algae, and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies most of the energy necessary for life on Earth.

The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. Carbon is the main component of biological compounds as well as a major component of many minerals such as limestone. Along with the nitrogen cycle and the water cycle, the carbon cycle comprises a sequence of events that are key to make Earth capable of sustaining life. It describes the movement of carbon as it is recycled and reused throughout the biosphere, as well as long-term processes of carbon sequestration to and release from carbon sinks. Carbon sinks in the land and the ocean each currently take up about one-quarter of anthropogenic carbon emissions each year.
In chemistry, carbonic acid is a dibasic acid with the chemical formula H2CO3. The pure compound decomposes at temperatures greater than ca. −80 °C.

Hypercapnia (from the Greek hyper = "above" or "too much" and kapnos = "smoke"), also known as hypercarbia and CO2 retention, is a condition of abnormally elevated carbon dioxide (CO2) levels in the blood. Carbon dioxide is a gaseous product of the body's metabolism and is normally expelled through the lungs. Carbon dioxide may accumulate in any condition that causes hypoventilation, a reduction of alveolar ventilation (the clearance of air from the small sacs of the lung where gas exchange takes place). Inability of the lungs to clear carbon dioxide leads to respiratory acidosis. Eventually the body compensates for the raised acidity by retaining alkali in the kidneys, a process known as "metabolic compensation".

Carbonic maceration is a winemaking technique, often associated with the French wine region of Beaujolais, in which whole grapes are fermented in a carbon dioxide rich environment before crushing. Conventional alcoholic fermentation involves crushing the grapes to free the juice and pulp from the skin with yeast serving to convert sugar into ethanol. Carbonic maceration ferments most of the juice while it is still inside the grape, although grapes at the bottom of the vessel are crushed by gravity and undergo conventional fermentation. The resulting wine is fruity with very low tannins. It is ready to drink quickly but lacks the structure for long-term aging. In extreme cases such as Beaujolais nouveau, the period between picking and bottling can be less than six weeks.
Hypocapnia, also known as hypocarbia, sometimes incorrectly called acapnia, is a state of reduced carbon dioxide in the blood. Hypocapnia usually results from shallow or rapid breathing, known as hyperventilation.

Charles David Keeling was an American scientist whose recording of carbon dioxide at the Mauna Loa Observatory confirmed Svante Arrhenius's proposition (1896) of the possibility of anthropogenic contribution to the "greenhouse effect" and global warming, by documenting the steadily rising carbon dioxide levels. The Keeling Curve measures the progressive buildup of carbon dioxide, a greenhouse gas, in the atmosphere.

Carbon capture and storage (CCS) or carbon capture and sequestration, is the process of capturing waste carbon dioxide (CO
2), transporting it to a storage site, and depositing it where it will not enter the atmosphere. Usually the CO2 is captured from large point sources, such as a cement factory or biomass power plant, and then stored in an underground geological formation. The aim is to prevent the release of large quantities of CO
2 into the atmosphere from heavy industry with the intent of mitigating the effects of climate change. Although CO
2 has been injected into geological formations for several decades for various purposes, including enhanced oil recovery, the long-term storage of CO
2 is a relatively new concept.
Enhanced oil recovery, also called tertiary recovery, is the extraction of crude oil from an oil field that cannot be extracted otherwise. EOR can extract 30% to 60% or more of a reservoir's oil, compared to 20% to 40% using primary and secondary recovery. According to the US Department of Energy, carbon dioxide and water are injected along with one of three EOR techniques: thermal injection, gas injection, and chemical injection. More advanced, speculative EOR techniques are sometimes called quaternary recovery.
The Haldane effect is a property of hemoglobin first described by John Scott Haldane, within which oxygenation of blood in the lungs displaces carbon dioxide from hemoglobin, increasing the removal of carbon dioxide. Consequently, oxygenated blood has a reduced affinity for carbon dioxide. Thus, the Haldane effect describes the ability of hemoglobin to carry increased amounts of carbon dioxide (CO2) in the deoxygenated state as opposed to the oxygenated state. A high concentration of CO2 facilitates dissociation of oxyhemoglobin.

Carbon sequestration is the long-term removal, capture or sequestration of carbon dioxide from the atmosphere to slow or reverse atmospheric CO2 pollution and to mitigate or reverse global warming.
Massachusetts v. Environmental Protection Agency, 549 U.S. 497 (2007), is a 5-4 U.S. Supreme Court case in which twelve states and several cities of the United States, represented by James Milkey, brought suit against the Environmental Protection Agency (EPA) to force that federal agency to regulate carbon dioxide and other greenhouse gases (GHGs) as pollutants.

Carbon trioxide (CO3) is an unstable oxide of carbon (an oxocarbon). The possible isomers of carbon trioxide include ones with molecular symmetry point groups Cs, D3h, and C2v. The C2v state, consisting of a dioxirane has been shown to be the ground state of the molecule. Carbon trioxide should not be confused with the stable carbonate ion (CO32−).

A Diet Coke and Mentos Eruption is a reaction between the carbonated beverage Diet Coke and Mentos mints that causes the beverage to expel from its container. The candies catalyze the release of gas from the beverage, which creates an eruption that pushes most of the liquid up and out of the bottle. Lee Marek and "Marek's Kid Scientists" were the first to publicly demonstrate the experiment on the Late Show with David Letterman in 1999. Steve Spangler's televised demonstration of the eruption in 2005 became popular on YouTube, launching a chain of several other Diet Coke and Mentos experiment viral videos. Experiments carried out at altitudes ranging from below sea level in Death Valley to the summit of Pikes Peak have demonstrated that the reaction works better at higher elevations.
A carbon dioxide scrubber is a piece of equipment that absorbs carbon dioxide (CO2). It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers. Carbon dioxide scrubbers are also used in controlled atmosphere (CA) storage. They have also been researched for carbon capture and storage as a means of combating global warming.
Work of breathing (WOB) is the energy expended to inhale and exhale a breathing gas. It is usually expressed as work per unit volume, for example, joules/litre, or as a work rate (power), such as joules/min or equivalent units, as it is not particularly useful without a reference to volume or time. It can be calculated in terms of the pulmonary pressure multiplied by the change in pulmonary volume, or in terms of the oxygen consumption attributable to breathing. In a normal resting state the work of breathing constitutes about 5% of the total body oxygen consumption. It can increase considerably due to illness or constraints on gas flow imposed by breathing apparatus, ambient pressure, or breathing gas composition.