 | |
| Editor | George Billman |
|---|---|
| Genre | Medicine |
| Publisher | John Wiley and Sons |
Publication date | 2010 |
Novel Therapeutic Targets for Antiarrhythmic Drugs is a book edited by George Billman and published by John Wiley and Sons in 2010.
 | |
| Editor | George Billman |
|---|---|
| Genre | Medicine |
| Publisher | John Wiley and Sons |
Publication date | 2010 |
Novel Therapeutic Targets for Antiarrhythmic Drugs is a book edited by George Billman and published by John Wiley and Sons in 2010.
According to the publisher, the book describes the current state of cardiac arrhythmia treatment, and attempts to identify future directions research may take. [1] Its 21 chapters cover a variety of topics related to cardiac arrhythmia and electrophysiology, primarily reviewing known molecular targets for drugs. [2] Subjects covered in the book include both traditional approaches to looking at arrhythmia, such as ion channel effects, and more general issues such as the genetics behind differential response to existing drug therapies. [3] Drug safety and side effects are also cover. [2]
The book examines avenues by which new treatments might be developed, with four chapters (10, 13, 16, 17) specifically focused on novel targets. [2] Novel ideas offered included studying sodium-calcium exchanger and ryanodine receptor effects. [3] One chapter (5) is dedicated to examining the targets on which existing drugs operate, and another (8) examines drugs in clinical trial at the time of publication. The chemical structure of existing drugs is not covered. [2] Overall, the book advocates for segregating drug targets by disease type and state, rather than the conventional approach of segregating by likelihood to harm. [3] In addition to pharmacological therapies, the book examines potential alternate treatments to arrhythmia including the effect of endurance training on susceptibility. It also investigates omega-3 fatty acids which have a proven effect on cardiac electrophysiology, but have failed to prove protective when obtained through diet. [3]
According to cardiologist Peter R. Kowey, the chapter authors are "eminent scientists" in their respective areas. [3] The list includes "some brilliant industry scientists", but does not include any clinicians or drug trialists, possibly creating a biased perspective, according to Kowey.
In a review for Circulation, Kowey called the book "an admirable attempt" to develop a more targeted approach to arrhythmia treatment, and said it was "illuminating and far reaching". [3] He particularly liked the advocated approach of segregating drug targets by disease state. Kowey said the book's main weakness was a lack of focus on clinical issues – both in topics covered and author selection. He noted that bringing drugs to the marketplace is expensive and proof of concept clinical trials are necessary to justify the investment. He said "Billman should be congratulated for his willingness to take on what is clearly an extraordinarily complex problem area" and praised him for "[encouraging] blue sky thinking" is his contributions to the book. [3] He also congratulated the chapter authors for making "complex discussions not only interpretable, but topical". [3] Kowey concluded "I would recommend this book to my colleagues and fellows, not only as a reference source, but as a compendium of information that summarizes where we are, and most importantly, the path we must take." [3]
In a review for ChemMedChem , medicinal chemistry professor Ahmed S. Mehanna agreed that Novel Therapeutic Targets for Antiarrhythmic Drugs did not adequately cover clinical aspects of drug development. He also said the book could have been better organized and focused too heavily on describing known research, as opposed to the novel treatments implied by the book's title. He did, however, say the book "gives very valuable and comprehensive reviews of arrhythmia and its pharmacological management." [2] Mehanna said the book reviewed topics appropriately and was free of obvious errors. Overall, he recommended the book, calling it "a worthwhile addition to the literature on cardiac arrhythmia and antiarrhythmic drugs." [2]
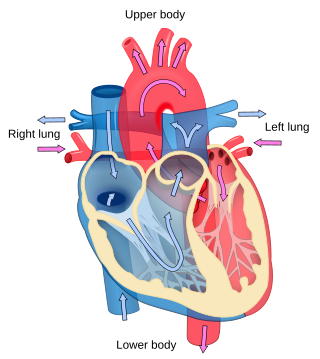
Cardiology is the study of the heart. Cardiology is a branch of medicine that deals with disorders of the heart and the cardiovascular system. The field includes medical diagnosis and treatment of congenital heart defects, coronary artery disease, heart failure, valvular heart disease, and electrophysiology. Physicians who specialize in this field of medicine are called cardiologists, a sub-specialty of internal medicine. Pediatric cardiologists are pediatricians who specialize in cardiology. Physicians who specialize in cardiac surgery are called cardiothoracic surgeons or cardiac surgeons, a specialty of general surgery.
Antiarrhythmic agents, also known as cardiac dysrhythmia medications, are a class of drugs that are used to suppress abnormally fast rhythms (tachycardias), such as atrial fibrillation, supraventricular tachycardia and ventricular tachycardia.

Quinidine is a class IA antiarrhythmic agent used to treat heart rhythm disturbances. It is a diastereomer of antimalarial agent quinine, originally derived from the bark of the cinchona tree. The drug causes increased action potential duration, as well as a prolonged QT interval. As of 2019, its IV formulation is no longer being manufactured for use in the United States.

Flecainide is a medication used to prevent and treat abnormally fast heart rates. This includes ventricular and supraventricular tachycardias. Its use is only recommended in those with dangerous arrhythmias or when significant symptoms cannot be managed with other treatments. Its use does not decrease a person's risk of death. It is taken by mouth or injection into a vein.
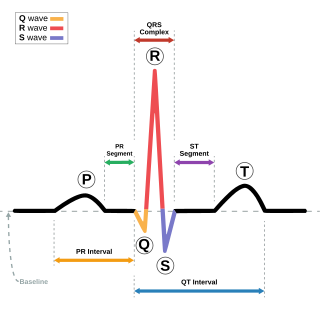
Cardiac electrophysiology is a branch of cardiology and basic science focusing on the electrical activities of the heart. The term is usually used in clinical context, to describe studies of such phenomena by invasive (intracardiac) catheter recording of spontaneous activity as well as of cardiac responses to programmed electrical stimulation - clinical cardiac electrophysiology. However, cardiac electrophysiology also encompasses basic research and translational research components. Specialists studying cardiac electrophysiology, either clinically or solely through research, are known as cardiac electrophysiologists.

Procainamide (PCA) is a medication of the antiarrhythmic class used for the treatment of cardiac arrhythmias. It is a sodium channel blocker of cardiomyocytes; thus it is classified by the Vaughan Williams classification system as class Ia. In addition to blocking the INa current, it inhibits the IKr rectifier K+ current. Procainamide is also known to induce a voltage-dependent open channel block on the batrachotoxin (BTX)-activated sodium channels in cardiomyocytes.

Catheter ablation is a procedure that uses radio-frequency energy or other sources to terminate or modify a faulty electrical pathway from sections of the heart of those who are prone to developing cardiac arrhythmias such as atrial fibrillation, atrial flutter and Wolff-Parkinson-White syndrome. If not controlled, such arrhythmias increase the risk of ventricular fibrillation and sudden cardiac arrest. The ablation procedure can be classified by energy source: radiofrequency ablation and cryoablation.

Vanoxerine is an investigational drug which is being evaluated for the treatment of heart arrhythmias and cocaine dependence. Vanoxerine is a piperazine derivative which has multiple pharmacological activities including acting as an dopamine reuptake inhibitor, serotonin transporter inhibitor, and as a blocker of the cardiac hERG repolarizing potassium channel (IKr).

Prajmaline (Neo-gilurythmal) is a class Ia antiarrhythmic agent which has been available since the 1970s. Class Ia drugs increase the time one action potential lasts in the heart. Prajmaline is a semi-synthetic propyl derivative of ajmaline, with a higher bioavailability than its predecessor. It acts to stop arrhythmias of the heart through a frequency-dependent block of cardiac sodium channels.

Lorcainide is a Class 1c antiarrhythmic agent that is used to help restore normal heart rhythm and conduction in patients with premature ventricular contractions, ventricular tachycardiac and Wolff–Parkinson–White syndrome. Lorcainide was developed by Janssen Pharmaceutica (Belgium) in 1968 under the commercial name Remivox and is designated by code numbers R-15889 or Ro 13-1042/001. It has a half-life of 8.9 +- 2.3 hrs which may be prolonged to 66 hrs in people with cardiac disease.
Clinical cardiac electrophysiology, is a branch of the medical specialty of cardiology concerned with the study and treatment of rhythm disorders of the heart. Cardiologists with expertise in this area are usually referred to as electrophysiologists. Electrophysiologists are trained in the mechanism, function, and performance of the electrical activities of the heart. Electrophysiologists work closely with other cardiologists and cardiac surgeons to assist or guide therapy for heart rhythm disturbances (arrhythmias). They are trained to perform interventional and surgical procedures to treat cardiac arrhythmia.

Dronedarone, sold under the brand name Multaq, is a class III antiarrhythmic medication developed by Sanofi-Aventis. It was approved by the US Food and Drug Administration (FDA) in July 2009. Besides being indicated in arrhythmias, it was recommended as an alternative to amiodarone for the treatment of atrial fibrillation and atrial flutter in people whose hearts have either returned to normal rhythm or who undergo drug therapy or electric shock treatment i.e. direct current cardioversion (DCCV) to maintain normal rhythm. It is a class III antiarrhythmic drug. The FDA label includes a claim for reducing hospitalization, but not for reducing mortality, as a reduction in mortality was not demonstrated in the clinical development program. A trial of the drug in heart failure was stopped as an interim analysis showed a possible increase in heart failure deaths, in people with moderate to severe congestive heart failure.

Michel Haïssaguerre is a French cardiologist and electrophysiologist. His investigations have been the basis for development of new markers and therapies for atrial and ventricular fibrillation.
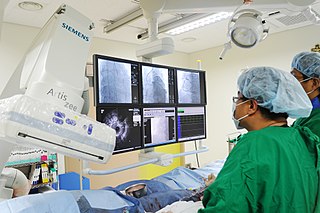
A cardiac electrophysiology study is a minimally invasive procedure using catheters introduced through a vein or artery to record electrical activity from within the heart. This electrical activity is recorded when the heart is in a normal rhythm to assess the conduction system of the heart and to look for additional electrical connections, and during any abnormal heart rhythms that can be induced. EP studies are used to investigate the cause, location of origin, and best treatment for various abnormal heart rhythms, and are often followed by a catheter ablation during the same procedure.

Henrick Joan Joost Wellens, M.D., (1935–2020) was a Dutch cardiologist who is considered one of the founding fathers of clinical cardiac electrophysiology - a discipline which enables patients with cardiac arrhythmias to have catheter electrode mapping and ablation.

BRL-32872 is an experimental drug candidate that provides a novel approach to the treatment of cardiac arrhythmia. Being a derivative of verapamil, it possesses the ability to inhibit Ca+2 membrane channels. Specific modifications in hydrogen bonding activity, nitrogen lone pair availability, and molecular flexibility allow BRL-32872 to inhibit K+ channels as well. As such, BRL-32872 is classified as both a class III (K+ blocking) and class IV (Ca+2 blocking) antiarrhythmic agent.

Celivarone is an experimental drug being tested for use in pharmacological antiarrhythmic therapy. Cardiac arrhythmia is any abnormality in the electrical activity of the heart. Arrhythmias range from mild to severe, sometimes causing symptoms like palpitations, dizziness, fainting, and even death. They can manifest as slow (bradycardia) or fast (tachycardia) heart rate, and may have a regular or irregular rhythm.

Budiodarone (ATI-2042) is an antiarrhythmic agent and chemical analog of amiodarone that is currently being studied in clinical trials. Amiodarone is considered the most effective antiarrhythmic drug available, but its adverse side effects, including hepatic, pulmonary and thyroid toxicity as well as multiple drug interactions, are discouraging its use. Budiodarone only differs in structure from amiodarone through the presence of a sec-butyl acetate side chain at position 2 of the benzofuran moiety. This side chain allows for budiodarone to have a shorter half-life in the body than amiodarone which allows it to have a faster onset of action and metabolism while still maintaining similar electrophysiological activity. The faster metabolism of budiodarone allows for fewer adverse side effects than amiodarone principally due to decreased levels of toxicity in the body.

Peter R. Kowey is an American cardiologist and medical researcher. He is Professor of Medicine and Clinical Pharmacology at Jefferson Medical College of Thomas Jefferson University and holds the William Wikoff Smith Chair in Cardiovascular Research at Lankenau Institute for Medical Research.
George Edward Billman is an American physiologist and professor at Ohio State University. After receiving a Ph.D from the University of Kentucky in 1980, Billman began his professional career at the University of Oklahoma. In 1984, he joined the Ohio State staff, where he became an associate professor in 1990 and a full professor in 1996.