Related Research Articles

In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group (−OH) bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethanol, which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest examples, includes all compounds which conform to the general formula CnH2n+1OH. Simple monoalcohols that are the subject of this article include primary, secondary and tertiary alcohols.

Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula C
2H
4 or H2C=CH2. It is a colorless flammable gas with a faint "sweet and musky" odor when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Weathering is the deterioration of rocks, soils and minerals as well as wood and artificial materials through contact with water, atmospheric gases, and biological organisms. Weathering occurs in situ, and so is distinct from erosion, which involves the transport of rocks and minerals by agents such as water, ice, snow, wind, waves and gravity.

Ethylene glycol is an organic compound with the formula (CH2OH)2. It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, sweet-tasting, flammable, viscous liquid. Ethylene glycol is toxic to humans in high concentrations.

Ethylene oxide is an organic compound with the formula C
2H
4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of silver catalyst.

An epoxide is a cyclic ether with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile.

Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, that dissolves in water as K+ and MnO−
4, an intensely pink to purple solution.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.

Total organic carbon (TOC) is the amount of carbon found in an organic compound and is often used as a non-specific indicator of water quality or cleanliness of pharmaceutical manufacturing equipment. TOC may also refer to the amount of organic carbon in soil, or in a geological formation, particularly the source rock for a petroleum play; 2% is a rough minimum. For marine surface sediments average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins.

Methyl methacrylate (MMA) is an organic compound with the formula CH2=C(CH3)COOCH3. This colorless liquid, the methyl ester of methacrylic acid (MAA), is a monomer produced on a large scale for the production of poly(methyl methacrylate) (PMMA).

Calcium sulfide is the chemical compound with the formula CaS. This white material crystallizes in cubes like rock salt. CaS has been studied as a component in a process that would recycle gypsum, a product of flue-gas desulfurization. Like many salts containing sulfide ions, CaS typically has an odour of H2S, which results from small amount of this gas formed by hydrolysis of the salt.

A carbonate ester (organic carbonate or organocarbonate) is an ester of carbonic acid. This functional group consists of a carbonyl group flanked by two alkoxy groups. The general structure of these carbonates is R1O(C=O)OR2 and they are related to esters R1O(C=O)R, ethers R1OR2 and also to the inorganic carbonates.

Dimethyl carbonate (DMC) is an organic compound with the formula OC(OCH3)2. It is a colourless, flammable liquid. It is classified as a carbonate ester. This compound has found use as a methylating agent and more recently as a solvent that is exempt from the restrictions placed on most volatile organic compounds (VOCs) in the US. Dimethyl carbonate is often considered to be a green reagent.

Ethylene carbonate (sometimes abbreviated EC) is the organic compound with the formula (CH2O)2CO. It is classified as the cyclic carbonate ester of ethylene glycol and carbonic acid. At room temperature (25 °C) ethylene carbonate is a transparent crystalline solid, practically odorless and colorless, and somewhat soluble in water. In the liquid state (m.p. 34-37 °C) it is a colorless odorless liquid.
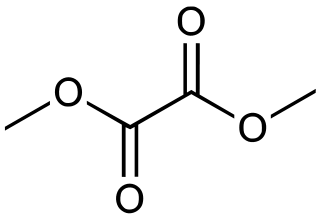
Dimethyl oxalate is the organic compound with the formula (CO2CH3)2. It is the dimethyl ester of oxalic acid. Dimethyl oxalate is a colorless or white solid that is soluble in water.
A carbon dioxide scrubber is a piece of equipment that absorbs carbon dioxide (CO2). It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers. Carbon dioxide scrubbers are also used in controlled atmosphere (CA) storage. They have also been researched for carbon capture and storage as a means of combating climate change.

The oxidation state of oxygen is −2 in almost all known compounds of oxygen. The oxidation state −1 is found in a few compounds such as peroxides. Compounds containing oxygen in other oxidation states are very uncommon: −1⁄2 (superoxides), −1⁄3 (ozonides), 0, +1⁄2 (dioxygenyl), +1, and +2.

Acetic acid, systematically named ethanoic acid, is an acidic, colourless liquid and organic compound with the chemical formula CH3COOH. Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements.
The oxidative coupling of methane (OCM) is a chemical reaction discovered in the 1980s for the direct conversion of natural gas, primarily consisting of methane, into value-added chemicals. The OCM process has not been commercially practiced.
References
- 1 2 "OMEGA delivers for ethylene glycol makers". Shell Chemicals. October 2008. Retrieved 2012-12-22.
- 1 2 3 4 Peter Taffe (2008-08-12). "Shell's Omega MEG process kicks off in South Korea". ICIS.com. Archived from the original on 2008-10-23.
- ↑ "First start-up of Shell OMEGA process plant" (Press release). Shell Chemicals. 2008-06-16.
- ↑ "Second Shell OMEGA process plant starts up in Saudi Arabia" (Press release). Shell Chemicals. 2009-06-02.
- ↑ "Shell starts-up world-scale monoethylene glycol plant in Singapore" (Press release). Shell Chemicals. 2009-11-17.