Related Research Articles

Phenylketonuria (PKU) is an inborn error of metabolism that results in decreased metabolism of the amino acid phenylalanine. Untreated PKU can lead to intellectual disability, seizures, behavioral problems, and mental disorders. It may also result in a musty smell and lighter skin. A baby born to a mother who has poorly treated PKU may have heart problems, a small head, and low birth weight.

Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown of purine nucleotides, and it is a normal component of urine. High blood concentrations of uric acid can lead to gout and are associated with other medical conditions, including diabetes and the formation of ammonium acid urate kidney stones.
Iron poisoning typically occurs from ingestion of excess iron that results in acute toxicity. Mild symptoms which occur within hours include vomiting, diarrhea, abdominal pain, and drowsiness. In more severe cases, symptoms can include tachypnea, low blood pressure, seizures, or coma. If left untreated, iron poisoning can lead to multi-organ failure resulting in permanent organ damage or death.
An arterial blood gas (ABG) test, or arterial blood gas analysis (ABGA) measures the amounts of arterial gases, such as oxygen and carbon dioxide. An ABG test requires that a small volume of blood be drawn from the radial artery with a syringe and a thin needle, but sometimes the femoral artery in the groin or another site is used. The blood can also be drawn from an arterial catheter.

Acetazolamide, sold under the trade name Diamox among others, is a medication used to treat glaucoma, epilepsy, acute mountain sickness, periodic paralysis, idiopathic intracranial hypertension, heart failure and to alkalinize urine. It may be used long term for the treatment of open angle glaucoma and short term for acute angle closure glaucoma until surgery can be carried out. It is taken by mouth or injection into a vein. Acetazolamide is a first generation carbonic anhydrase inhibitor and it decreases the ocular fluid and osmolality in the eye to decrease intraocular pressure.
Acidosis is a process causing increased acidity in the blood and other body tissues. If not further qualified, it usually refers to acidity of the blood plasma.

Chelation therapy is a medical procedure that involves the administration of chelating agents to remove heavy metals from the body. Chelation therapy has a long history of use in clinical toxicology and remains in use for some very specific medical treatments, although it is administered under very careful medical supervision due to various inherent risks, including the mobilization of mercury and other metals through the brain and other parts of the body by the use of weak chelating agents that unbind with metals before elimination, exacerbating existing damage. To avoid mobilization, some practitioners of chelation use strong chelators, such as selenium, taken at low doses over a long period of time.

Metabolic acidosis is a serious electrolyte disorder characterized by an imbalance in the body's acid-base balance. Metabolic acidosis has three main root causes: increased acid production, loss of bicarbonate, and a reduced ability of the kidneys to excrete excess acids. Metabolic acidosis can lead to acidemia, which is defined as arterial blood pH that is lower than 7.35. Acidemia and acidosis are not mutually exclusive – pH and hydrogen ion concentrations also depend on the coexistence of other acid-base disorders; therefore, pH levels in people with metabolic acidosis can range from low to high.

Megaloblastic anemia is a type of macrocytic anemia. An anemia is a red blood cell defect that can lead to an undersupply of oxygen. Megaloblastic anemia results from inhibition of DNA synthesis during red blood cell production. When DNA synthesis is impaired, the cell cycle cannot progress from the G2 growth stage to the mitosis (M) stage. This leads to continuing cell growth without division, which presents as macrocytosis. Megaloblastic anemia has a rather slow onset, especially when compared to that of other anemias. The defect in red cell DNA synthesis is most often due to hypovitaminosis, specifically vitamin B12 deficiency or folate deficiency. Loss of micronutrients may also be a cause.
Transaminases or aminotransferases are enzymes that catalyze a transamination reaction between an amino acid and an α-keto acid. They are important in the synthesis of amino acids, which form proteins.

Metabolic alkalosis is a metabolic condition in which the pH of tissue is elevated beyond the normal range (7.35–7.45). This is the result of decreased hydrogen ion concentration, leading to increased bicarbonate, or alternatively a direct result of increased bicarbonate concentrations. The condition typically cannot last long if the kidneys are functioning properly.
In physiology, base excess and base deficit refer to an excess or deficit, respectively, in the amount of base present in the blood. The value is usually reported as a concentration in units of mEq/L (mmol/L), with positive numbers indicating an excess of base and negative a deficit. A typical reference range for base excess is −2 to +2 mEq/L.

Alkaline diet describes a group of loosely related diets based on the misconception that different types of food can have an effect on the pH balance of the body. It originated from the acid ash hypothesis, which primarily related to osteoporosis research. Proponents of the diet believe that certain foods can affect the acidity (pH) of the body and that the change in pH can therefore be used to treat or prevent disease. However, their claims are false, and there is no evidence supporting the claimed mechanisms of this diet, which is not recommended by dietitians or other health professionals.

β-Hydroxybutyric acid, also known as 3-hydroxybutyric acid or BHB, is an organic compound and a beta hydroxy acid with the chemical formula CH3CH(OH)CH2CO2H; its conjugate base is β-hydroxybutyrate, also known as 3-hydroxybutyrate. β-Hydroxybutyric acid is a chiral compound with two enantiomers: D-β-hydroxybutyric acid and L-β-hydroxybutyric acid. Its oxidized and polymeric derivatives occur widely in nature. In humans, D-β-hydroxybutyric acid is one of two primary endogenous agonists of hydroxycarboxylic acid receptor 2 (HCA2), a Gi/o-coupled G protein-coupled receptor (GPCR).
Acid–base homeostasis is the homeostatic regulation of the pH of the body's extracellular fluid (ECF). The proper balance between the acids and bases in the ECF is crucial for the normal physiology of the body—and for cellular metabolism. The pH of the intracellular fluid and the extracellular fluid need to be maintained at a constant level.

Peter Arthur Robert Stewart (1921–1993) was a Canadian physiologist who introduced an alternate approach to understanding acid–base physiology.

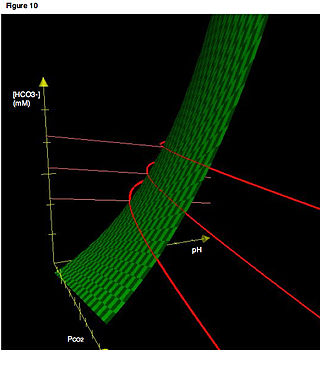
The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid (H2CO3), bicarbonate ion (HCO−
3), and carbon dioxide (CO2) in order to maintain pH in the blood and duodenum, among other tissues, to support proper metabolic function. Catalyzed by carbonic anhydrase, carbon dioxide (CO2) reacts with water (H2O) to form carbonic acid (H2CO3), which in turn rapidly dissociates to form a bicarbonate ion (HCO−
3 ) and a hydrogen ion (H+) as shown in the following reaction:
Winters' formula, named after Dr. R.W. Winters, is a formula used to evaluate respiratory compensation when analyzing acid-base disorders in the presence of metabolic acidosis. It can be given as:

The Illawarra Health and Medical Research Institute (IHMRI) is an independent health and medical research institute based in Wollongong, New South Wales.
Alexander Haig was a Scottish physician, dietitian and vegetarianism activist. He was best known for pioneering the uric-acid free diet.
References
- ↑ Siggaard-Andersen, Ole; Engel K (1960). "A new acid-base nomogram. An improved method for the calculation of the relevant blood acid-base data". Scan J Clin Lab Invest. 12 (2): 177–86. doi:10.3109/00365516009062420. PMID 13848805.
- 1 2 "Ole Siggaard-Andersen, PhD". Archived from the original on 2009-06-21. Retrieved 2009-09-21.