Related Research Articles

In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose–Einstein condensates, neutron-degenerate matter, and quark–gluon plasma, which only occur, respectively, in situations of extreme cold, extreme density, and extremely high energy. For a complete list of all exotic states of matter, see the list of states of matter.

In chemistry, thermodynamics, and many other related fields, phase transitions are the physical processes of transition between a state of a medium, identified by some parameters, and another one, with different values of the parameters. Commonly the term is used to refer to changes among the basic states of matter: solid, liquid, and gas, as well as plasma in rare cases.

An amorphous metal is a solid metallic material, usually an alloy, with disordered atomic-scale structure. Most metals are crystalline in their solid state, which means they have a highly ordered arrangement of atoms. Amorphous metals are non-crystalline, and have a glass-like structure. But unlike common glasses, such as window glass, which are typically electrical insulators, amorphous metals have good electrical conductivity and they also display superconductivity at low temperatures.
In physics, the terms order and disorder designate the presence or absence of some symmetry or correlation in a many-particle system.
Amorphous ice is an amorphous solid form of water. Common ice is a crystalline material wherein the molecules are regularly arranged in a hexagonal lattice, whereas amorphous ice has a lack of long-range order in its molecular arrangement. Amorphous ice is produced either by rapid cooling of liquid water, or by compressing ordinary ice at low temperatures.
In chemistry, the study of sonochemistry is concerned with understanding the effect of ultrasound in forming acoustic cavitation in liquids, resulting in the initiation or enhancement of the chemical activity in the solution. Therefore, the chemical effects of ultrasound do not come from a direct interaction of the ultrasonic sound wave with the molecules in the solution.

A spin ice is a magnetic substance that does not have a single minimal-energy state. It has magnetic moments (i.e. "spin") as elementary degrees of freedom which are subject to frustrated interactions. By their nature, these interactions prevent the moments from exhibiting a periodic pattern in their orientation down to a temperature much below the energy scale set by the said interactions. Spin ices show low-temperature properties, residual entropy in particular, closely related to those of common crystalline water ice. The most prominent compounds with such properties are dysprosium titanate (Dy2Ti2O7) and holmium titanate (Ho2Ti2O7). The orientation of the magnetic moments in spin ice resembles the positional organization of hydrogen atoms (more accurately, ionized hydrogen, or protons) in conventional water ice (see figure 1).
Liquid crystal polymers (LCPs) are polymers with the property of liquid crystal, usually containing aromatic rings as mesogens. Despite uncrosslinked LCPs, polymeric materials like liquid crystal elastomers (LCEs) and liquid crystal networks (LCNs) can exhibit liquid crystallinity as well. They are both crosslinked LCPs but have different cross link density. They are widely used in the digital display market. In addition, LCPs have unique properties like thermal actuation, anisotropic swelling, and soft elasticity. Therefore, they can be good actuators and sensors. One of the most famous and classical applications for LCPs is Kevlar, a strong but light fiber with wide applications including bulletproof vests.
Indium(III) sulfate (In2(SO4)3) is a sulfate salt of the metal indium. It is a sesquisulfate, meaning that the sulfate group occurs 11/2 times as much as the metal. It may be formed by the reaction of indium, its oxide, or its carbonate with sulfuric acid. An excess of strong acid is required, otherwise insoluble basic salts are formed. As a solid indium sulfate can be anhydrous, or take the form of a pentahydrate with five water molecules or a nonahydrate with nine molecules of water. Indium sulfate is used in the production of indium or indium containing substances. Indium sulfate also can be found in basic salts, acidic salts or double salts including indium alum.

Zeolitic imidazolate frameworks (ZIFs) are a class of metal-organic frameworks (MOFs) that are topologically isomorphic with zeolites. ZIF glasses can be synthesized by the melt-quench method, and the first melt-quenched ZIF glass was firstly made and reported by Bennett et al. back in 2015. ZIFs are composed of tetrahedrally-coordinated transition metal ions connected by imidazolate linkers. Since the metal-imidazole-metal angle is similar to the 145° Si-O-Si angle in zeolites, ZIFs have zeolite-like topologies. As of 2010, 105 ZIF topologies have been reported in the literature. Due to their robust porosity, resistance to thermal changes, and chemical stability, ZIFs are being investigated for applications such as carbon dioxide capture.

A colloidal crystal is an ordered array of colloid particles and fine grained materials analogous to a standard crystal whose repeating subunits are atoms or molecules. A natural example of this phenomenon can be found in the gem opal, where spheres of silica assume a close-packed locally periodic structure under moderate compression. Bulk properties of a colloidal crystal depend on composition, particle size, packing arrangement, and degree of regularity. Applications include photonics, materials processing, and the study of self-assembly and phase transitions.
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification.
Crystallization of polymers is a process associated with partial alignment of their molecular chains. These chains fold together and form ordered regions called lamellae, which compose larger spheroidal structures named spherulites. Polymers can crystallize upon cooling from melting, mechanical stretching or solvent evaporation. Crystallization affects optical, mechanical, thermal and chemical properties of the polymer. The degree of crystallinity is estimated by different analytical methods and it typically ranges between 10 and 80%, with crystallized polymers often called "semi-crystalline". The properties of semi-crystalline polymers are determined not only by the degree of crystallinity, but also by the size and orientation of the molecular chains.
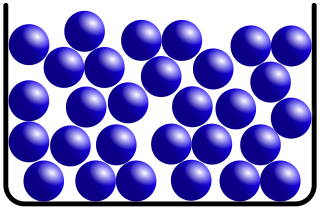
The structure of liquids, glasses and other non-crystalline solids is characterized by the absence of long-range order which defines crystalline materials. Liquids and amorphous solids do, however, possess a rich and varied array of short to medium range order, which originates from chemical bonding and related interactions. Metallic glasses, for example, are typically well described by the dense random packing of hard spheres, whereas covalent systems, such as silicate glasses, have sparsely packed, strongly bound, tetrahedral network structures. These very different structures result in materials with very different physical properties and applications.
Splat quenching is a metallurgical, metal morphing technique used for forming metals with a particular crystal structure by means of extremely rapid quenching, or cooling.
Rigidity theory, or topological constraint theory, is a tool for predicting properties of complex networks based on their composition. It was introduced by James Charles Phillips in 1979 and 1981, and refined by Michael Thorpe in 1983. Inspired by the study of the stability of mechanical trusses as pioneered by James Clerk Maxwell, and by the seminal work on glass structure done by William Houlder Zachariasen, this theory reduces complex molecular networks to nodes constrained by rods, thus filtering out microscopic details that ultimately don't affect macroscopic properties. An equivalent theory was developed by P.K. Gupta A.R. Cooper in 1990, where rather than nodes representing atoms, they represented unit polytopes. An example of this would be the SiO tetrahedra in pure glassy silica. This style of analysis has applications in biology and chemistry, such as understanding adaptability in protein-protein interaction networks. Rigidity theory applied to the molecular networks arising from phenotypical expression of certain diseases may provide insights regarding their structure and function.

Solid nitrogen is a number of solid forms of the element nitrogen, first observed in 1884. Solid nitrogen is mainly the subject of academic research, but low-temperature, low-pressure solid nitrogen is a substantial component of bodies in the outer Solar System and high-temperature, high-pressure solid nitrogen is a powerful explosive, with higher energy density than any other non-nuclear material.
Hyperuniform materials are mixed-component many-particle systems with unusually low fluctuations in component density at large scales, when compared to the distribution of constituents in common disordered systems, like a mixed ideal gas (air) or typical liquids or amorphous solids: A disordered hyperuniform system is statistically isotropic, like a liquid, but exhibits reduced long-wavelength density fluctuations, similar to crystals.

Isaac B. Bersuker is a Soviet-Moldоvan-American theoretical physicist and quantum chemist whose principal research is in chemical physics, solid-state physics, and theoretical chemistry. Known for his "life-long years of experience in theoretical chemistry" working on the electronic structure and properties of coordination compounds, Isaac B. Bersuker is “one of the most widely recognized authorities” in the theory of the Jahn-Teller effect (JTE) and the pseudo-Jahn-Teller effect (PJTE). His accomplishments include explaining the polarization of the atomic core in Rydberg atoms, the effect of tunneling splitting in molecules and solids with a strong JTE, and the discovery of the PJTE origin of ferroelectricity in cubic perovskites. Known as the leading expert in JTE and PJTE, Bersuker is the permanent Chairman of the International Steering Committee of the Jahn-Teller symposia. His present affiliation is with the Oden Institute for Computational Engineering and Science of the Department of Chemistry of the University of Texas at Austin.
Caesium sesquioxide is a chemical compound with the formula Cs2O3 or Cs4O6. In terms of oxidation states, Caesium in this compound has a nominal charge of +1, and the oxygen is a mixed peroxide (O22-) and superoxide (O2−) for a structural formula of (Cs+)4(O2−)2(O22-). Compared to the other caesium oxides, this phase is less well studied, but has been long present in the literature. It can be created by thermal decomposition of caesium superoxide at 290 °C.
References
- ↑ Loidl, A. (1989). "Orientational Glasses". Annual Review of Physical Chemistry. Annual Reviews. 40 (1): 29–60. Bibcode:1989ARPC...40...29L. doi:10.1146/annurev.pc.40.100189.000333. ISSN 0066-426X.
- ↑ Moura Ramos, Joaquim J.; Diogo, Hermínio P. (2019). "Orientational glass, orientationally disordered crystal and crystalline polymorphism: A further study on the thermal behavior and molecular mobility in levoglucosan". Journal of Molecular Liquids. Elsevier BV. 286: 110914. doi:10.1016/j.molliq.2019.110914. ISSN 0167-7322. S2CID 164662415.
- ↑ Knorr, K (1987-01-01). "The Glasslike State of the Mixed Alkali Halide-Alkali Cyanides". Physica Scripta. IOP Publishing. T19B: 531–536. Bibcode:1987PhST...19..531K. doi:10.1088/0031-8949/1987/t19b/034. ISSN 0031-8949.