
The enzyme-linked immunosorbent assay (ELISA) is a commonly used analytical biochemistry assay, first described by Eva Engvall and Peter Perlmann in 1971. The assay is a solid-phase type of enzyme immunoassay (EIA) to detect the presence of a ligand in a liquid sample using antibodies directed against the ligand to be measured. ELISA has been used as a diagnostic tool in medicine, plant pathology, and biotechnology, as well as a quality control check in various industries.

HIV tests are used to detect the presence of the human immunodeficiency virus (HIV), the virus that causes acquired immunodeficiency syndrome (AIDS), in serum, saliva, or urine. Such tests may detect antibodies, antigens, or RNA.
Serology is the scientific study of serum and other body fluids. In practice, the term usually refers to the diagnostic identification of antibodies in the serum. Such antibodies are typically formed in response to an infection, against other foreign proteins, or to one's own proteins. In either case, the procedure is simple.

Chiron Corporation was an American multinational biotechnology firm founded in 1981, based in Emeryville, California, that was acquired by Novartis on April 20, 2006. It had offices and facilities in eighteen countries on five continents. Chiron's business and research was in three main areas: biopharmaceuticals, vaccines, and blood testing. Chiron's vaccines and blood testing units were combined to form Novartis Vaccines and Diagnostics, while Chiron BioPharmaceuticals was integrated into Novartis Pharmaceuticals. In 2014, Novartis completed the sale of its blood transfusion diagnostics unit to Grifols and announced agreements for the sale of its vaccines unit to GlaxoSmithKline.
The direct and indirect Coombs tests, also known as antiglobulin test (AGT), are blood tests used in immunohematology. The direct Coombs test detects antibodies that are stuck to the surface of the red blood cells. Since these antibodies sometimes destroy red blood cells they can cause anemia; this test can help clarify the condition. The indirect Coombs test detects antibodies that are floating freely in the blood. These antibodies could act against certain red blood cells; the test can be carried out to diagnose reactions to a blood transfusion.

Immunohematology is a branch of hematology and transfusion medicine which studies antigen-antibody reactions and analogous phenomena as they relate to the pathogenesis and clinical manifestations of blood disorders. A person employed in this field is referred to as an immunohematologist or colloquially as a blood banker. Their day-to-day duties include blood typing, cross-matching and antibody identification.
Interferon-gamma release assays (IGRAs) are diagnostic tools for latent tuberculosis infection (LTBI). They are surrogate markers of Mycobacterium tuberculosis infection and indicate a cellular immune response to M. tuberculosis if the latter is present.

A medical laboratory or clinical laboratory is a laboratory where tests are conducted out on clinical specimens to obtain information about the health of a patient to aid in diagnosis, treatment, and prevention of disease. Clinical medical laboratories are an example of applied science, as opposed to research laboratories that focus on basic science, such as found in some academic institutions.
This page is currently under construction.
Immudex is a Danish Reagents and Diagnostics company established in 2009. The company is operating from offices located in Copenhagen, Denmark, and in Fairfax, Virginia. Immudex specializes in the production of MHC Dextramers. MHC Dextramers are chemical reagents that are designed to detect antigen-specific T cells.
DiaSorin is an Italian multinational biotechnology company that produces and markets in vitro diagnostics reagent kits used in immunodiagnostics and molecular diagnostics and since July 2021, it is also active in the Life Science business. The group was founded in 2000 and is headquartered in Saluggia, Italy. Its production is at several plants located in Europe and the United States: Saluggia and Gerenzano (Italy), Dietzenbach (Germany), Stillwater, Minnesota (US), Dartford (UK). Following the acquisition of Luminex, the company acquired five additional production plants located in the United States and in Canada (Toronto). The company is a constituent of the FTSE MIB index.
QuidelOrtho Corporation is an American manufacturer of diagnostic healthcare products that are sold worldwide.
Viral load monitoring for HIV is the regular measurement of the viral load of individual HIV-positive people as part of their personal plan for treatment of HIV/AIDS. A count of the viral load is routine before the start of HIV treatment.

David Maurice Surrey Dane, MRCS CRCP MB Bchir MRCP MRCPath FRCPath FRCP was a pre-eminent British pathologist and clinical virologist known for his pioneering work in infectious diseases including poliomyelitis and the early investigations into the efficacy of a number of vaccines. He is particularly remembered for his strategic foresight in the field of blood transfusion microbiology, particularly in relation to diseases that are spread through blood transfusion.
The Vel blood group is a human blood group that has been implicated in hemolytic transfusion reactions. The blood group consists of a single antigen, the high-frequency Vel antigen, which is expressed on the surface of red blood cells. Individuals are typed as Vel-positive or Vel-negative depending on the presence of this antigen. The expression of the antigen in Vel-positive individuals is highly variable and can range from strong to weak. Individuals with the rare Vel-negative blood type develop anti-Vel antibodies when exposed to Vel-positive blood, which can cause transfusion reactions on subsequent exposures.
The Junior blood group system is a human blood group defined by the presence or absence of the Jr(a) antigen, a high-frequency antigen that is found on the red blood cells of most individuals. People with the rare Jr(a) negative blood type can develop anti-Jr(a) antibodies, which may cause transfusion reactions and hemolytic disease of the newborn on subsequent exposures. Jr(a) negative blood is most common in people of Japanese heritage.

Blood compatibility testing is conducted in a medical laboratory to identify potential incompatibilities between blood group systems in blood transfusion. It is also used to diagnose and prevent some complications of pregnancy that can occur when the baby has a different blood group from the mother. Blood compatibility testing includes blood typing, which detects the antigens on red blood cells that determine a person's blood type; testing for unexpected antibodies against blood group antigens ; and, in the case of blood transfusions, mixing the recipient's plasma with the donor's red blood cells to detect incompatibilities (crossmatching). Routine blood typing involves determining the ABO and RhD type, and involves both identification of ABO antigens on red blood cells and identification of ABO antibodies in the plasma. Other blood group antigens may be tested for in specific clinical situations.
The Lan blood group system is a human blood group defined by the presence or absence of the Lan antigen on a person's red blood cells. More than 99.9% of people are positive for the Lan antigen. Individuals with the rare Lan-negative blood type, which is a recessive trait, can produce an anti-Lan antibody when exposed to Lan-positive blood. Anti-Lan antibodies may cause transfusion reactions on subsequent exposures to Lan-positive blood, and have also been implicated in mild cases of hemolytic disease of the newborn. However, the clinical significance of the antibody is variable. The antigen was first described in 1961, and Lan was officially designated a blood group in 2012.
The WHO model list of essential in vitro diagnostics, or WHO list of essential diagnostic tests (EDL) is a World Health Organization (WHO) priority list of medical tests that provides guidance for individual countries on which tests to use and which not to. It was first published in 2018, then revised in 2019, and a third edition was published in 2020.
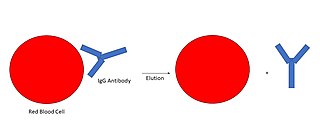
An antibody elution is a clinical laboratory diagnostic procedure which removes sensitized antibodies from red blood cells, in order to determine the blood group system antigen the antibody targets. An antibody elution is deemed necessary when antibodies of the immunoglobulin class G (IgG) are found sensitized (bound) to peripheral red cells collected from a blood product transfusion recipient. IgG antibodies are detected using an assay known as the direct antiglobulin test.







