
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Ligands can bind either to extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed.

Integrins are transmembrane receptors that facilitate cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface.
A hormone receptor is a receptor molecule that binds to a specific hormone. Hormone receptors are a wide family of proteins made up of receptors for thyroid and steroid hormones, retinoids and Vitamin D, and a variety of other receptors for various ligands, such as fatty acids and prostaglandins. There are two main classes of hormone receptors. Receptors for peptide hormones tend to be cell surface receptors built into the plasma membrane of cells and are thus referred to as trans membrane receptors. An example of this is insulin. Receptors for steroid hormones are usually found within the cytoplasm and are referred to as intracellular or nuclear receptors, such as testosterone. Upon hormone binding, the receptor can initiate multiple signaling pathways, which ultimately leads to changes in the behavior of the target cells.

In biochemistry and pharmacology, receptors are chemical structures, composed of protein, that receive and transduce signals that may be integrated into biological systems. These signals are typically chemical messengers which bind to a receptor and cause some form of cellular/tissue response, e.g. a change in the electrical activity of a cell. There are three main ways the action of the receptor can be classified: relay of signal, amplification, or integration. Relaying sends the signal onward, amplification increases the effect of a single ligand, and integration allows the signal to be incorporated into another biochemical pathway.

Abscisic acid (ABA) is a plant hormone. ABA functions in many plant developmental processes, including seed and bud dormancy, the control of organ size and stomatal closure. It is especially important for plants in the response to environmental stresses, including drought, soil salinity, cold tolerance, freezing tolerance, heat stress and heavy metal ion tolerance.
Steroid hormone receptors are found in the nucleus, cytosol, and also on the plasma membrane of target cells. They are generally intracellular receptors and initiate signal transduction for steroid hormones which lead to changes in gene expression over a time period of hours to days. The best studied steroid hormone receptors are members of the nuclear receptor subfamily 3 (NR3) that include receptors for estrogen and 3-ketosteroids. In addition to nuclear receptors, several G protein-coupled receptors and ion channels act as cell surface receptors for certain steroid hormones.

Selective estrogen receptor modulators (SERMs), also known as estrogen receptor agonist/antagonists (ERAAs), are a class of drugs that act on the estrogen receptor (ER). A characteristic that distinguishes these substances from pure ER agonists and antagonists is that their action is different in various tissues, thereby granting the possibility to selectively inhibit or stimulate estrogen-like action in various tissues.

Ligand-gated ion channels (LICs, LGIC), also commonly referred to as ionotropic receptors, are a group of transmembrane ion-channel proteins which open to allow ions such as Na+, K+, Ca2+, and/or Cl− to pass through the membrane in response to the binding of a chemical messenger (i.e. a ligand), such as a neurotransmitter.

Estrogen receptors (ERs) are a group of proteins found inside cells. They are receptors that are activated by the hormone estrogen (17β-estradiol). Two classes of ER exist: nuclear estrogen receptors, which are members of the nuclear receptor family of intracellular receptors, and membrane estrogen receptors (mERs), which are mostly G protein-coupled receptors. This article refers to the former (ER).
Second messengers are intracellular signaling molecules released by the cell in response to exposure to extracellular signaling molecules—the first messengers. Second messengers trigger physiological changes at cellular level such as proliferation, differentiation, migration, survival, apoptosis and depolarization.

In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from ligare, which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. The instance of binding occurs over an infinitesimal range of time and space, so the rate constant is usually a very small number.
In biology, cell signaling or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. It is a fundamental property of all cells in every living organism such as bacteria, plants, and animals. Signals that originate from outside a cell can be physical agents like mechanical pressure, voltage, temperature, light, or chemical signals. Chemical signals can be hydrophobic or hydrophillic. Cell signaling can occur over short or long distances, and as a result can be classified as autocrine, juxtacrine, intracrine, paracrine, or endocrine. Signaling molecules can be synthesized from various biosynthetic pathways and released through passive or active transports, or even from cell damage.
The thyroid hormone receptor (TR) is a type of nuclear receptor that is activated by binding thyroid hormone. TRs act as transcription factors, ultimately affecting the regulation of gene transcription and translation. These receptors also have non-genomic effects that lead to second messenger activation, and corresponding cellular response.
The transforming growth factor beta (TGFB) signaling pathway is involved in many cellular processes in both the adult organism and the developing embryo including cell growth, cell differentiation, cell migration. apoptosis, cellular homeostasis and other cellular functions. This TGFB signaling pathways are conserved. In spite of the wide range of cellular processes that the TGFβ signaling pathway regulates, the process is relatively simple. TGFβ superfamily ligands bind to a type II receptor, which recruits and phosphorylates a type I receptor. The type I receptor then phosphorylates receptor-regulated SMADs (R-SMADs) which can now bind the coSMAD SMAD4. R-SMAD/coSMAD complexes accumulate in the nucleus where they act as transcription factors and participate in the regulation of target gene expression.

The luteinizing hormone/choriogonadotropin receptor (LHCGR), also lutropin/choriogonadotropin receptor (LCGR) or luteinizing hormone receptor (LHR) is a transmembrane receptor found predominantly in the ovary and testis, but also many extragonadal organs such as the uterus and breasts. The receptor interacts with both luteinizing hormone (LH) and chorionic gonadotropins and represents a G protein-coupled receptor (GPCR). Its activation is necessary for the hormonal functioning during reproduction.
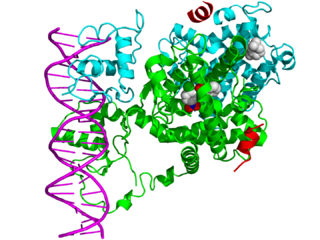
In the field of molecular biology, nuclear receptors are a class of proteins found within cells that are responsible for sensing steroid and thyroid hormones and certain other molecules. In response, these receptors work with other proteins to regulate the expression of specific genes, thereby controlling the development, homeostasis, and metabolism of the organism.

Chemokine receptors are cytokine receptors found on the surface of certain cells that interact with a type of cytokine called a chemokine. There have been 20 distinct chemokine receptors discovered in humans. Each has a rhodopsin-like 7-transmembrane (7TM) structure and couples to G-protein for signal transduction within a cell, making them members of a large protein family of G protein-coupled receptors. Following interaction with their specific chemokine ligands, chemokine receptors trigger a flux in intracellular calcium (Ca2+) ions (calcium signaling). This causes cell responses, including the onset of a process known as chemotaxis that traffics the cell to a desired location within the organism. Chemokine receptors are divided into different families, CXC chemokine receptors, CC chemokine receptors, CX3C chemokine receptors and XC chemokine receptors that correspond to the 4 distinct subfamilies of chemokines they bind. Four families of chemokine receptors differ in spacing of cysteine residues near N-terminal of the receptor.
Siglecs(Sialic acid-binding immunoglobulin-type lectins) are cell surface proteins that bind sialic acid. They are found primarily on the surface of immune cells and are a subset of the I-type lectins. There are 14 different mammalian Siglecs, providing an array of different functions based on cell surface receptor-ligand interactions.

Cell surface receptors are receptors that are embedded in the plasma membrane of cells. They act in cell signaling by receiving extracellular molecules. They are specialized integral membrane proteins that allow communication between the cell and the extracellular space. The extracellular molecules may be hormones, neurotransmitters, cytokines, growth factors, cell adhesion molecules, or nutrients; they react with the receptor to induce changes in the metabolism and activity of a cell. In the process of signal transduction, ligand binding affects a cascading chemical change through the cell membrane.

The bump-and-hole method is a tool in chemical genetics for studying a specific isoform in a protein family without perturbing the other members of the family. The unattainability of isoform-selective inhibition due to structural homology in protein families is a major challenge of chemical genetics. With the bump-and-hole approach, a protein–ligand interface is engineered to achieve selectivity through steric complementarity while maintaining biochemical competence and orthogonality to the wild type pair. Typically, a "bumped" ligand/inhibitor analog is designed to bind a corresponding "hole-modified" protein. Bumped ligands are commonly bulkier derivatives of a cofactor of the target protein. Hole-modified proteins are recombinantly expressed with an amino acid substitution from a larger to smaller residue, e.g. glycine or alanine, at the cofactor binding site. The designed ligand/inhibitor has specificity for the engineered protein due to steric complementarity, but not the native counterpart due to steric interference.













