
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane. It is also defined as the measure of the tendency of a solution to take in its pure solvent by osmosis. Potential osmotic pressure is the maximum osmotic pressure that could develop in a solution if it were separated from its pure solvent by a semipermeable membrane.

Semipermeable membrane is a type of synthetic or biologic, polymeric membrane that allows certain molecules or ions to pass through it by osmosis. The rate of passage depends on the pressure, concentration, and temperature of the molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability may depend on solute size, solubility, properties, or chemistry. How the membrane is constructed to be selective in its permeability will determine the rate and the permeability. Many natural and synthetic materials which are rather thick are also semipermeable. One example of this is the thin film on the inside of an egg.
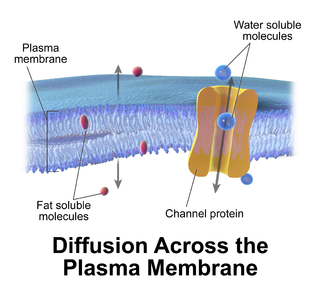
Passive transport is a type of membrane transport that does not require energy to move substances across cell membranes. Instead of using cellular energy, like active transport, passive transport relies on the second law of thermodynamics to drive the movement of substances across cell membranes. Fundamentally, substances follow Fick's first law, and move from an area of high concentration to an area of low concentration because this movement increases the entropy of the overall system. The rate of passive transport depends on the permeability of the cell membrane, which, in turn, depends on the organization and characteristics of the membrane lipids and proteins. The four main kinds of passive transport are simple diffusion, facilitated diffusion, filtration, and/or osmosis.
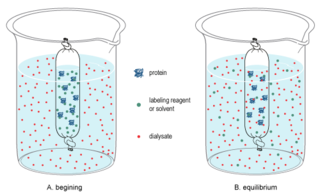
In chemistry, dialysis is the process of separating molecules in solution by the difference in their rates of diffusion through a semipermeable membrane, such as dialysis tubing.

Forward osmosis (FO) is an osmotic process that, like reverse osmosis (RO), uses a semi-permeable membrane to effect separation of water from dissolved solutes. The driving force for this separation is an osmotic pressure gradient, such that a "draw" solution of high concentration, is used to induce a net flow of water through the membrane into the draw solution, thus effectively separating the feed water from its solutes. In contrast, the reverse osmosis process uses hydraulic pressure as the driving force for separation, which serves to counteract the osmotic pressure gradient that would otherwise favor water flux from the permeate to the feed. Hence significantly more energy is required for reverse osmosis compared to forward osmosis.
Water potential is the potential energy of water per unit volume relative to pure water in reference conditions. Water potential quantifies the tendency of water to move from one area to another due to osmosis, gravity, mechanical pressure and matrix effects such as capillary action. The concept of water potential has proved useful in understanding and computing water movement within plants, animals, and soil. Water potential is typically expressed in potential energy per unit volume and very often is represented by the Greek letter ψ.

Electrophoretic deposition (EPD), is a term for a broad range of industrial processes which includes electrocoating, cathodic electrodeposition, anodic electrodeposition, and electrophoretic coating, or electrophoretic painting. A characteristic feature of this process is that colloidal particles suspended in a liquid medium migrate under the influence of an electric field (electrophoresis) and are deposited onto an electrode. All colloidal particles that can be used to form stable suspensions and that can carry a charge can be used in electrophoretic deposition. This includes materials such as polymers, pigments, dyes, ceramics and metals.

A primer or undercoat is a preparatory coating put on materials before painting. Priming ensures better adhesion of paint to the surface, increases paint durability, and provides additional protection for the material being painted.

In chemical biology, tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a partially-permeable cell membrane. Tonicity depends on the relative concentration of selective membrane-impermeable solutes across a cell membrane which determine the direction and extent of osmotic flux. It is commonly used when describing the swelling-versus-shrinking response of cells immersed in an external solution.

Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution. The osmolarity of a solution is usually expressed as Osm/L, in the same way that the molarity of a solution is expressed as "M". Whereas molarity measures the number of moles of solute per unit volume of solution, osmolarity measures the number of osmoles of solute particles per unit volume of solution. This value allows the measurement of the osmotic pressure of a solution and the determination of how the solvent will diffuse across a semipermeable membrane (osmosis) separating two solutions of different osmotic concentration.
Turgor pressure is the force within the cell that pushes the plasma membrane against the cell wall.

Osmotic shock or osmotic stress is physiologic dysfunction caused by a sudden change in the solute concentration around a cell, which causes a rapid change in the movement of water across its cell membrane. Under hypertonic conditions - conditions of high concentrations of either salts, substrates or any solute in the supernatant - water is drawn out of the cells through osmosis. This also inhibits the transport of substrates and cofactors into the cell thus “shocking” the cell. Alternatively, under hypotonic conditions - when concentrations of solutes are low - water enters the cell in large amounts, causing it to swell and either burst or undergo apoptosis.

Decorative concrete is the use of concrete as not simply a utilitarian medium for construction but as an aesthetic enhancement to a structure, while still serving its function as an integral part of the building itself such as floors, walls, driveways, and patios.

Nanofiltration is a membrane filtration process that uses nanometer sized pores through which particles smaller than about 1–10 nanometers pass through the membrane. Nanofiltration membranes have pore sizes of about 1–10 nanometers, smaller than those used in microfiltration and ultrafiltration, but a slightly bigger than those in reverse osmosis. Membranes used are predominantly polymer thin films. It is used to soften, disinfect, and remove impurities from water, and to purify or separate chemicals such as pharmaceuticals.
Reverse osmosis (RO) is a water purification process that uses a semi-permeable membrane to separate water molecules from other substances. RO applies pressure to overcome osmotic pressure that favors even distributions. RO can remove dissolved or suspended chemical species as well as biological substances, and is used in industrial processes and the production of potable water. RO retains the solute on the pressurized side of the membrane and the purified solvent passes to the other side. The relative sizes of the various molecules determines what passes through. "Selective" membranes reject large molecules, while accepting smaller molecules.

Osmosis is the spontaneous net movement or diffusion of solvent molecules through a selectively-permeable membrane from a region of high water potential to a region of low water potential, in the direction that tends to equalize the solute concentrations on the two sides. It may also be used to describe a physical process in which any solvent moves across a selectively permeable membrane separating two solutions of different concentrations. Osmosis can be made to do work. Osmotic pressure is defined as the external pressure required to prevent net movement of solvent across the membrane. Osmotic pressure is a colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity.
Osmoregulation is the active regulation of the osmotic pressure of an organism's body fluids, detected by osmoreceptors, to maintain the homeostasis of the organism's water content; that is, it maintains the fluid balance and the concentration of electrolytes to keep the body fluids from becoming too diluted or concentrated. Osmotic pressure is a measure of the tendency of water to move into one solution from another by osmosis. The higher the osmotic pressure of a solution, the more water tends to move into it. Pressure must be exerted on the hypertonic side of a selectively permeable membrane to prevent diffusion of water by osmosis from the side containing pure water.

The Bresle method is used to determine concentration of soluble salts on metal surfaces prior to coating application, such as painting. These salts can cause serious adhesion problems after time.
Concrete sealers are applied to concrete to protect it from surface damage, corrosion, and staining. They either block the pores in the concrete to reduce absorption of water and salts or form an impermeable layer which prevents such materials from passing.
Biomaterials exhibit various degrees of compatibility with the harsh environment within a living organism. They need to be nonreactive chemically and physically with the body, as well as integrate when deposited into tissue. The extent of compatibility varies based on the application and material required. Often modifications to the surface of a biomaterial system are required to maximize performance. The surface can be modified in many ways, including plasma modification and applying coatings to the substrate. Surface modifications can be used to affect surface energy, adhesion, biocompatibility, chemical inertness, lubricity, sterility, asepsis, thrombogenicity, susceptibility to corrosion, degradation, and hydrophilicity.













