
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Ligands can bind either to extracellular N-terminus and loops or to the binding site within transmembrane helices. They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed.

Neisseria is a large genus of bacteria that colonize the mucosal surfaces of many animals. Of the 11 species that colonize humans, only two are pathogens, N. meningitidis and N. gonorrhoeae. Most gonococcal infections are asymptomatic and self-resolving, and epidemic strains of the meningococcus may be carried in >95% of a population where systemic disease occurs at <1% prevalence.
The restriction modification system is found in bacteria and other prokaryotic organisms, and provides a defense against foreign DNA, such as that borne by bacteriophages.

Lipopolysaccharides (LPS) are large molecules consisting of a lipid and a polysaccharide composed of O-antigen, outer core and inner core joined by a covalent bond; they are found in the outer membrane of Gram-negative bacteria. The term lipooligosaccharide ("LOS") is used to refer to a low-molecular-weight form of bacterial lipopolysaccharides.

The ATP-binding cassette transporters are a transport system superfamily that is one of the largest and possibly one of the oldest gene families. It is represented in all extant phyla, from prokaryotes to humans.
Cell adhesion molecules (CAMs) are a subset of cell adhesion proteins located on the cell surface involved in binding with other cells or with the extracellular matrix (ECM) in the process called cell adhesion. In essence, cell adhesion molecules help cells stick to each other and to their surroundings. Cell adhesion is a crucial component in maintaining tissue structure and function. In fully developed animals, these molecules play an integral role in creating force and movement and consequently ensure that organs are able to execute their functions. In addition to serving as "molecular glue", cell adhesion is important in affecting cellular mechanisms of growth, contact inhibition, and apoptosis. Oftentimes aberrant expression of CAMs will result in pathologies ranging from frostbite to cancer.

Neisseria meningitidis, often referred to as meningococcus, is a Gram-negative bacterium that can cause meningitis and other forms of meningococcal disease such as meningococcemia, a life-threatening sepsis. The bacterium is referred to as a coccus because it is round, and more specifically, a diplococcus because of its tendency to form pairs. About 10% of adults are carriers of the bacteria in their nasopharynx. As an exclusively human pathogen it is the main cause of bacterial meningitis in children and young adults, causing developmental impairment and death in about 10% of cases. It causes the only form of bacterial meningitis known to occur epidemically, mainly in Africa and Asia. It occurs worldwide in both epidemic and endemic form. N. meningitidis is spread through saliva and respiratory secretions during coughing, sneezing, kissing, chewing on toys and even through sharing a source of fresh water. It has also been reported to be transmitted through oral sex and cause urethritis in men. It infects its host cells by sticking to them with long thin extensions called pili and the surface-exposed proteins Opa and Opc and has several virulence factors.
Adhesins are cell-surface components or appendages of bacteria that facilitate adhesion or adherence to other cells or to surfaces, usually in the host they are infecting or living in. Adhesins are a type of virulence factor.
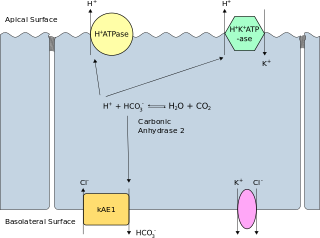
Band 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the SLC4A1 gene in humans.

Versican is a large extracellular matrix proteoglycan that is present in a variety of human tissues. It is encoded by the VCAN gene.
In biology, cell signaling or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. It is a fundamental property of all cells in every living organism such as bacteria, plants, and animals. Signals that originate from outside a cell can be physical agents like mechanical pressure, voltage, temperature, light, or chemical signals. Chemical signals can be hydrophobic or hydrophillic. Cell signaling can occur over short or long distances, and as a result can be classified as autocrine, juxtacrine, intracrine, paracrine, or endocrine. Signaling molecules can be synthesized from various biosynthetic pathways and released through passive or active transports, or even from cell damage.
Pilin refers to a class of fibrous proteins that are found in pilus structures in bacteria. These structures can be used for the exchange of genetic material, or as a cell adhesion mechanism. Although not all bacteria have pili or fimbriae, bacterial pathogens often use their fimbriae to attach to host cells. In Gram-negative bacteria, where pili are more common, individual pilin molecules are linked by noncovalent protein-protein interactions, while Gram-positive bacteria often have polymerized LPXTG pilin.

Syndecan 1 is a protein which in humans is encoded by the SDC1 gene. The protein is a transmembrane heparan sulfate proteoglycan and is a member of the syndecan proteoglycan family. The syndecan-1 protein functions as an integral membrane protein and participates in cell proliferation, cell migration and cell-matrix interactions via its receptor for extracellular matrix proteins. Syndecan-1 is a sponge for growth factors and chemokines, with binding largely via heparan sulfate chains. The syndecans mediate cell binding, cell signaling, and cytoskeletal organization and syndecan receptors are required for internalization of the HIV-1 tat protein.

Syndecan-2 is a protein that in humans is encoded by the SDC2 gene.

Syndecan-4 is a protein that in humans is encoded by the SDC4 gene. Syndecan-4 is one of the four vertebrate syndecans and has a molecular weight of ~20 kDa. Syndecans are the best-characterized plasma membrane proteoglycans. Their intracellular domain of membrane-spanning core protein interacts with actin cytoskeleton and signaling molecules in the cell cortex. Syndecans are normally found on the cell surface of fibroblasts and epithelial cells. Syndecans interact with fibronectin on the cell surface, cytoskeletal and signaling proteins inside the cell to modulate the function of integrin in cell-matrix adhesion. Also, syndecans bind to FGFs and bring them to the FGF receptor on the same cell. As a co-receptor or regulator, mutated certain proteoglycans could cause severe developmental defects, like disordered distribution or inactivation of signaling molecules.
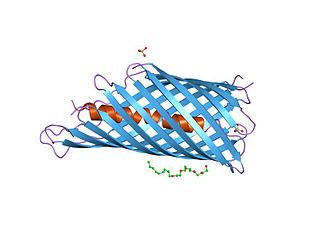
In molecular biology, an autotransporter domain is a structural domain found in some bacterial outer membrane proteins. The domain is always located at the C-terminal end of the protein and forms a beta-barrel structure. The barrel is oriented in the membrane such that the N-terminal portion of the protein, termed the passenger domain, is presented on the cell surface. These proteins are typically virulence factors, associated with infection or virulence in pathogenic bacteria.
Many bacteria secrete small iron-binding molecules called siderophores, which bind strongly to ferric ions. FepA is an integral bacterial outer membrane porin protein that belongs to outer membrane receptor family and provides the active transport of iron bound by the siderophore enterobactin from the extracellular space, into the periplasm of Gram-negative bacteria. FepA has also been shown to transport vitamin B12, and colicins B and D as well. This protein belongs to family of ligand-gated protein channels.

In molecular biology, trimeric autotransporter adhesins (TAAs), are proteins found on the outer membrane of Gram-negative bacteria. Bacteria use TAAs in order to infect their host cells via a process called cell adhesion. TAAs also go by another name, oligomeric coiled-coil adhesins, which is shortened to OCAs. In essence, they are virulence factors, factors that make the bacteria harmful and infective to the host organism.

In molecular biology, YadA is a protein domain which is short for Yersinia adhesin A. These proteins have strong sequence and structural homology, particularly at their C-terminal end. The function is to promote their pathogenicity and virulence in host cells, though cell adhesion. YadA is found in three pathogenic species of Yersinia, Y. pestis,Y. pseudotuberculosis, and Y. enterocolitica. The YadA domain is encoded for by a virulence plasmid in Yersinia, which encodes a type-III secretion (T3S) system consisting of the Ysc injectisome and the Yop effectors.

In 1928, Neisseria flavescens was first isolated from cerebrospinal fluid in the midst of an epidemic meningitis outbreak in Chicago. These gram-negative, aerobic bacteria reside in the mucosal membranes of the upper respiratory tract, functioning as commensals. However, this species can also play a pathogenic role in immunocompromised and diabetic individuals. In rare cases, it has been linked to meningitis, pneumonia, empyema, endocarditis, and sepsis.














