
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula HO2C−CO2H. It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis, commonly known as wood-sorrels. It occurs naturally in many foods. Excessive ingestion of oxalic acid or prolonged skin contact can be dangerous.

Pyridinium refers to the cation [C5H5NH]+. It is the conjugate acid of pyridine. Many related cations are known involving substituted pyridines, e.g. picolines, lutidines, collidines. They are prepared by treating pyridine with acids.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.

Oxalyl chloride is an organic chemical compound with the formula (COCl)2. This colorless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis.

A trimethylsilyl group (abbreviated TMS) is a functional group in organic chemistry. This group consists of three methyl groups bonded to a silicon atom [−Si(CH3)3], which is in turn bonded to the rest of a molecule. This structural group is characterized by chemical inertness and a large molecular volume, which makes it useful in a number of applications.
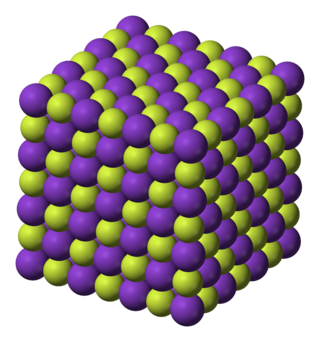
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali halide and occurs naturally as the rare mineral carobbiite. Solutions of KF will etch glass due to the formation of soluble fluorosilicates, although HF is more effective.

Iron(II) fluoride or ferrous fluoride is an inorganic compound with the molecular formula FeF2. It forms a tetrahydrate FeF2·4H2O that is often referred to by the same names. The anhydrous and hydrated forms are white crystalline solids.
An inorganic nonaqueous solvent is a solvent other than water, that is not an organic compound. These solvents are used in chemical research and industry for reactions that cannot occur in aqueous solutions or require a special environment. Inorganic nonaqueous solvents can be classified into two groups, protic solvents and aprotic solvents. Early studies on inorganic nonaqueous solvents evaluated ammonia, hydrogen fluoride, sulfuric acid, as well as more specialized solvents, hydrazine, and selenium oxychloride.
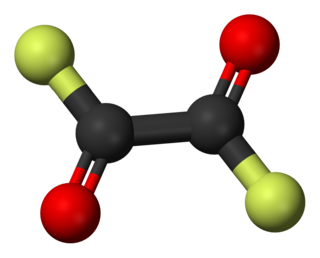
Oxalyl fluoride is the organofluorine compound with the formula (COF)2. It is a fluorinated derivative of oxalic acid. This colorless liquid is prepared by reaction of sodium fluoride with oxalyl chloride.

Thiazyl fluoride, NSF, is a colourless, pungent gas at room temperature and condenses to a pale yellow liquid at 0.4 °C. Along with thiazyl trifluoride, NSF3, it is an important precursor to sulfur-nitrogen-fluorine compounds. It is notable for its extreme hygroscopicity.

Oxalic anhydride or ethanedioic anhydride, also called oxiranedione, is a hypothetical organic compound, one of several isomers having the formula C2O3 that have been studied computationally. It can be viewed as the anhydride of oxalic acid or the two-fold ketone of ethylene oxide. It is an oxide of carbon (an oxocarbon).

Dioxane tetraketone (or 1,4-dioxane-2,3,5,6-tetrone) is an organic compound with the formula C4O6. It is an oxide of carbon (an oxocarbon), which can be viewed as the fourfold ketone of dioxane. It can also be viewed as the cyclic dimer of oxiranedione (C2O3), the hypothetical anhydride of oxalic acid.

Tetrahydroxy-1,4-benzoquinone bisoxalate is a chemical compound, an oxide of carbon with formula C
10O
10. Its molecule consists of a 1,4-benzoquinone core with the four hydrogen atoms replaced by two oxalate groups. It can be seen as a fourfold ester of tetrahydroxy-1,4-benzoquinone and oxalic acid.

Vanadium(V) fluoride is the inorganic compound with the chemical formula VF5. It is a colorless volatile liquid. It is a highly reactive compound, as indicated by its ability to fluorinate organic substances.

Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.
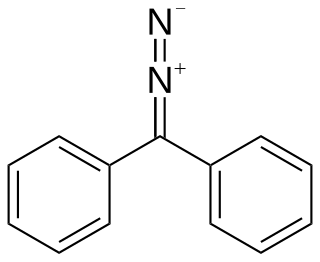
Diazodiphenylmethane is an organic reagent with the chemical formula C13H10N2. It exists as red-black crystals that melts just above room temperature.

Europium compounds are compounds formed by the lanthanide metal europium (Eu). In these compounds, europium generally exhibits the +3 oxidation state, such as EuCl3, Eu(NO3)3 and Eu(CH3COO)3. Compounds with europium in the +2 oxidation state are also known. The +2 ion of europium is the most stable divalent ion of lanthanide metals in aqueous solution. Lipophilic europium complexes often feature acetylacetonate-like ligands, e.g., Eufod.

The hexafluoroarsenate anion is a chemical species with formula AsF−6. Hexafluoroarsenate is relatively inert, being the conjugate base of the notional superacid hexafluoroarsenic acid.
Manganese(III) chloride is the hypothetical inorganic compound with the formula MnCl3.
















