
Calorimetry is the science or act of measuring changes in state variables of a body for the purpose of deriving the heat transfer associated with changes of its state due, for example, to chemical reactions, physical changes, or phase transitions under specified constraints. Calorimetry is performed with a calorimeter. The word calorimetry is derived from the Latin word calor, meaning heat and the Greek word μέτρον (metron), meaning measure. Scottish physician and scientist Joseph Black, who was the first to recognize the distinction between heat and temperature, is said to be the founder of the science of calorimetry.

Thermochemistry is the study of the heat energy which is associated with chemical reactions and/or physical transformations. A reaction may release or absorb energy, and a phase change may do the same, such as in melting and boiling. Thermochemistry focuses on these energy changes, particularly on the system's energy exchange with its surroundings. Thermochemistry is useful in predicting reactant and product quantities throughout the course of a given reaction. In combination with entropy determinations, it is also used to predict whether a reaction is spontaneous or non-spontaneous, favorable or unfavorable.

A calorimeter is an object used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. Differential scanning calorimeters, isothermal micro calorimeters, titration calorimeters and accelerated rate calorimeters are among the most common types. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. It is one of the measurement devices used in the study of thermodynamics, chemistry, and biochemistry.

Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. Generally, the temperature program for a DSC analysis is designed such that the sample holder temperature increases linearly as a function of time. The reference sample should have a well-defined heat capacity over the range of temperatures to be scanned.

Corrosion is a natural process that converts a refined metal into a more chemically stable form such as oxide, hydroxide, carbonate or sulfide. It is the gradual destruction of materials by chemical and/or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion.
Rancidification is the process of complete or incomplete oxidation or hydrolysis of fats and oils when exposed to air, light, or moisture or by bacterial action, resulting in unpleasant taste and odor. Specifically, it is the hydrolysis or autoxidation of fats into short-chain aldehydes, ketones and free fatty acids, which are objectionable in taste and odor. When these processes occur in food, undesirable odors and flavors can result.
Thermal analysis is a branch of materials science where the properties of materials are studied as they change with temperature. Several methods are commonly used – these are distinguished from one another by the property which is measured:

Thermogravimetric analysis or thermal gravimetric analysis (TGA) is a method of thermal analysis in which the mass of a sample is measured over time as the temperature changes. This measurement provides information about physical phenomena, such as phase transitions, absorption, adsorption and desorption; as well as chemical phenomena including chemisorptions, thermal decomposition, and solid-gas reactions.
Starch gelatinization is a process of breaking down the intermolecular bonds of starch molecules in the presence of water and heat, allowing the hydrogen bonding sites to engage more water. This irreversibly dissolves the starch granule in water. Water acts as a plasticizer.

Fouling is the accumulation of unwanted material on solid surfaces. The fouling materials can consist of either living organisms (biofouling) or a non-living substance. Fouling is usually distinguished from other surface-growth phenomena in that it occurs on a surface of a component, system, or plant performing a defined and useful function and that the fouling process impedes or interferes with this function.
Isothermal titration calorimetry (ITC) is a physical technique used to determine the thermodynamic parameters of interactions in solution. It is most often used to study the binding of small molecules to larger macromolecules . It consists of two cells which are enclosed in an adiabatic jacket. The compounds to be studied are placed in the sample cell, while the other cell, the reference cell, is used as a control and contains the buffer in which the sample is dissolved.
Thermomechanical analysis (TMA) is a technique used in thermal analysis, a branch of materials science which studies the properties of materials as they change with temperature.

Oxy-fuel welding and oxy-fuel cutting are processes that use fuel gases and oxygen to weld or cut metals. French engineers Edmond Fouché and Charles Picard became the first to develop oxygen-acetylene welding in 1903. Pure oxygen, instead of air, is used to increase the flame temperature to allow localized melting of the workpiece material in a room environment. A common propane/air flame burns at about 2,250 K, a propane/oxygen flame burns at about 2,526 K, an oxyhydrogen flame burns at 3,073 K and an acetylene/oxygen flame burns at about 3,773 K.
In polymers, such as plastics, thermal degradation refers to a type of polymer degradation where damaging chemical changes take place at elevated temperatures, without the simultaneous involvement of other compounds such as oxygen. Simply put, even in the absence of air, polymers will begin to degrade if heated high enough. It is distinct from thermal-oxidation, which can usually take place at less elevated temperatures.
Induction brazing is a process in which two or more materials are joined together by a filler metal that has a lower melting point than the base materials using induction heating. In induction heating, usually ferrous materials are heated rapidly from the electromagnetic field that is created by the alternating current from an induction coil.
Thermoporometry and cryoporometry are methods for measuring porosity and pore-size distributions. A small region of solid melts at a lower temperature than the bulk solid, as given by the Gibbs–Thomson equation. Thus, if a liquid is imbibed into a porous material, and then frozen, the melting temperature will provide information on the pore-size distribution. The detection of the melting can be done by sensing the transient heat flows during phase transitions using differential scanning calorimetry – DSC thermoporometry, measuring the quantity of mobile liquid using nuclear magnetic resonance – NMR cryoporometry (NMRC) or measuring the amplitude of neutron scattering from the imbibed crystalline or liquid phases – ND cryoporometry (NDC).
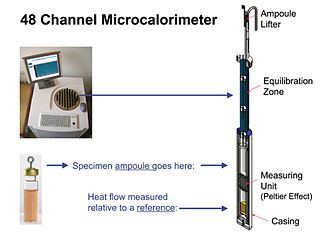
Isothermal microcalorimetry (IMC) is a laboratory method for real-time monitoring and dynamic analysis of chemical, physical and biological processes. Over a period of hours or days, IMC determines the onset, rate, extent and energetics of such processes for specimens in small ampoules at a constant set temperature.
The index of physics articles is split into multiple pages due to its size.
Accelerated testing of adhesives is used to obtain long term performance of an adhesive. Adhesives are commonly used for structural joints in various applications. Adhesives offer light weight and cost efficient options in design of mechanical members. In many applications, adhesive joints are the main load bearing joint as well as acting as a sealant in a joint. As such, the ability of adhesives for long service life needs to be assessed and evaluated. This is done based on the long term performance information available regarding a particular adhesive of choice. One of the ways to obtain the long term performance of an adhesive is using accelerated tests. In these tests possible failure mechanisms of an adhesive system will be simulated in a manner that accelerates time.








