Related Research Articles

The ATP-binding cassette transporters are a transport system superfamily that is one of the largest and possibly one of the oldest gene families. It is represented in all extant phyla, from prokaryotes to humans. ABC transporters belong to translocases.
The Transporter Classification Database is an International Union of Biochemistry and Molecular Biology (IUBMB)-approved classification system for membrane transport proteins, including ion channels.
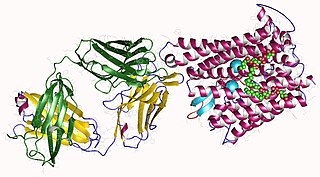
Ferroportin-1, also known as solute carrier family 40 member 1 (SLC40A1) or iron-regulated transporter 1 (IREG1), is a protein that in humans is encoded by the SLC40A1 gene, and is part of the Ferroportin (Fpn)Family. Ferroportin is a transmembrane protein that transports iron from the inside of a cell to the outside of the cell. Ferroportin is the only known iron exporter.
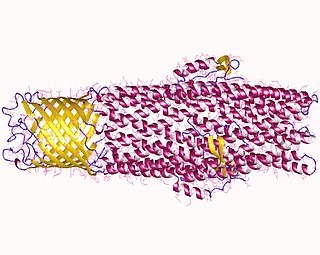
All microorganisms, with a few exceptions, have highly conserved DNA sequences in their genome that are transcribed and translated to efflux pumps. Efflux pumps are capable of moving a variety of different toxic compounds out of cells, such as antibiotics, heavy metals, organic pollutants, plant-produced compounds, quorum sensing signals, bacterial metabolites and neurotransmitters via active efflux, which is vital part for xenobiotic metabolism. This active efflux mechanism is responsible for various types of resistance to bacterial pathogens within bacterial species - the most concerning being antibiotic resistance because microorganisms can have adapted efflux pumps to divert toxins out of the cytoplasm and into extracellular media.
The solute carrier (SLC) group of membrane transport proteins include over 400 members organized into 66 families. Most members of the SLC group are located in the cell membrane. The SLC gene nomenclature system was originally proposed by the HUGO Gene Nomenclature Committee (HGNC) and is the basis for the official HGNC names of the genes that encode these transporters. A more general transmembrane transporter classification can be found in TCDB database.

Multidrug and toxin extrusion protein 1 (MATE1), also known as solute carrier family 47 member 1, is a protein that in humans is encoded by the SLC47A1 gene. SLC47A1 belongs to the MATE family of transporters that are found in bacteria, archaea and eukaryotes.

Multidrug and toxin extrusion protein 2 is a protein which in humans is encoded by the SLC47A2 gene.
Multi-antimicrobial extrusion protein (MATE) also known as multidrug and toxin extrusion or multidrug and toxic compound extrusion is a family of proteins which function as drug/sodium or proton antiporters.
Cation diffusion facilitators (CDFs) are transmembrane proteins that provide tolerance of cells to divalent metal ions, such as cadmium, zinc, and cobalt. These proteins are considered to be efflux pumps that remove these divalent metal ions from cells. However, some members of the CDF superfamily are implicated in ion uptake. All members of the CDF family possess six putative transmembrane spanners with strongest conservation in the four N-terminal spanners. The Cation Diffusion Facilitator (CDF) Superfamily includes the following families:

Neisseria gonorrhoeae, the bacterium that causes the sexually transmitted infection gonorrhea, has developed antibiotic resistance to many antibiotics. The bacteria was first identified in 1879.
The Amino Acid-Polyamine-Organocation (APC) Family of transport proteins includes members that function as solute:cation symporters and solute:solute antiporters. They occur in bacteria, archaea, fungi, unicellular eukaryotic protists, slime molds, plants and animals. They vary in length, being as small as 350 residues and as large as 850 residues. The smaller proteins are generally of prokaryotic origin while the larger ones are of eukaryotic origin. Most of them possess twelve transmembrane α-helical spanners but have a re-entrant loop involving TMSs 2 and 3. The APC Superfamily was established to encompass a wider range of homologues.
The multidrug/oligosaccharidyl-lipid/polysaccharide (MOP) flippase superfamily is a group of integral membrane protein families. The MOP flippase superfamily includes twelve distantly related families, six for which functional data are available:
- One ubiquitous family (MATE) specific for drugs - (TC# 2.A.66.1) The Multi Antimicrobial Extrusion (MATE) Family
- One (PST) specific for polysaccharides and/or their lipid-linked precursors in prokaryotes - (TC# 2.A.66.2) The Polysaccharide Transport (PST) Family
- One (OLF) specific for lipid-linked oligosaccharide precursors of glycoproteins in eukaryotes - (TC# 2.A.66.3) The Oligosaccharidyl-lipid Flippase (OLF) Family
- One (MVF) lipid-peptidoglycan precursor flippase involved in cell wall biosynthesis - (TC# 2.A.66.4) The Mouse Virulence Factor (MVF) Family
- One (AgnG) which includes a single functionally characterized member that extrudes the antibiotic, Agrocin 84 - (TC# 2.A.66.5) The Agrocin 84 Antibiotic Exporter (AgnG) Family
- And finally, one (Ank) that shuttles inorganic pyrophosphate (PPi) - (TC# 2.A.66.9) The Progressive Ankylosis (Ank) Family
The Manganese (Mn2+) Exporter (MntP) Family is a member of the Lysine Exporter (LysE) Superfamily. The MntP family is a small family whose members have been found in bacteria and archaea. MntP proteins are of about 200 amino acyl residues with 6 putative transmembrane segments (TMSs). The Conserved Domain Database (CDD) recognized two DUF204 repeats, each repeat having 3 TMSs. A representative list of proteins belonging to the MntP family can be found in the Transporter Classification Database.
The Tellurium Ion Resistance (TerC) Family is part of the Lysine Exporter (LysE) Superfamily. A representative list of proteins belonging to the TerC family can be found in the Transporter Classification Database.
The Nickel/Cobalt Transporter (NicO) Family is a member of the Lysine Exporter (LysE) Superfamily.
The ion transporter (IT) superfamily is a superfamily of secondary carriers that transport charged substrates.
The 6TMS Neutral Amino Acid Transporter (NAAT) Family is a family of transporters belonging to the Lysine Exporter (LysE) Superfamily. Homologues are found in numerous Gram-negative and Gram-positive bacteria including many human pathogens. Several archaea also encode MarC homologues. Some of these organisms have 2 or more paralogues. Most of these proteins are of about the same size although a few are larger. They exhibit 6 putative TMSs. A representative list of members belonging to the NAAT family can be found in the Transporter Classification Database.
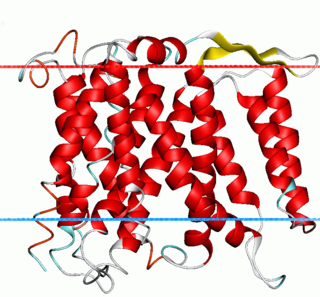
The Monovalent Cation:Proton Antiporter-1 (CPA1) Family (TC# 2.A.36) is a large family of proteins derived from Gram-positive and Gram-negative bacteria, blue-green bacteria, archaea, yeast, plants and animals. The CPA1 family belongs to the VIC superfamily. Transporters from eukaryotes have been functionally characterized to catalyze Na+:H+ exchange. Their primary physiological functions are thought to be in (1) cytoplasmic pH regulation, extruding the H+ generated during metabolism, and (2) salt tolerance (in plants), due to Na+ uptake into vacuoles. Bacterial homologues have also been found to facilitate Na+:H+ antiport, but some also catalyze Li+:H+ antiport or Ca2+:H+ antiport under certain conditions.

Resistance-nodulation-division (RND) family transporters are a category of bacterial efflux pumps, especially identified in Gram-negative bacteria and located in the cytoplasmic membrane, that actively transport substrates. The RND superfamily includes seven families: the heavy metal efflux (HME), the hydrophobe/amphiphile efflux-1, the nodulation factor exporter family (NFE), the SecDF protein-secretion accessory protein family, the hydrophobe/amphiphile efflux-2 family, the eukaryotic sterol homeostasis family, and the hydrophobe/amphiphile efflux-3 family. These RND systems are involved in maintaining homeostasis of the cell, removal of toxic compounds, and export of virulence determinants. They have a broad substrate spectrum and can lead to the diminished activity of unrelated drug classes if over-expressed. The first reports of drug resistant bacterial infections were reported in the 1940s after the first mass production of antibiotics. Most of the RND superfamily transport systems are made of large polypeptide chains. RND proteins exist primarily in gram-negative bacteria but can also be found in gram-positive bacteria, archaea, and eukaryotes.
The Reduced Folate Carrier (RFC) Family is a group of transport proteins that is part of the major facilitator superfamily. RFCs take up folate, reduced folate, derivatives of reduced folate and the drug, methotrexate.
References
- ↑ Prakash S, Cooper G, Singhi S, Saier MH (December 2003). "The ion transporter superfamily". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1618 (1): 79–92. doi: 10.1016/j.bbamem.2003.10.010 . PMID 14643936.
- ↑ Rabus R, Jack DL, Kelly DJ, Saier MH (December 1999). "TRAP transporters: an ancient family of extracytoplasmic solute-receptor-dependent secondary active transporters". Microbiology. 145 ( Pt 12) (12): 3431–45. doi: 10.1099/00221287-145-12-3431 . PMID 10627041.
- ↑ Hussein MJ, Green JM, Nichols BP (December 1998). "Characterization of mutations that allow p-aminobenzoyl-glutamate utilization by Escherichia coli". Journal of Bacteriology. 180 (23): 6260–8. doi:10.1128/JB.180.23.6260-6268.1998. PMC 107711 . PMID 9829935.
- 1 2 Folster JP, Shafer WM (June 2005). "Regulation of mtrF expression in Neisseria gonorrhoeae and its role in high-level antimicrobial resistance". Journal of Bacteriology. 187 (11): 3713–20. doi:10.1128/JB.187.11.3713-3720.2005. PMC 1112036 . PMID 15901695.
- 1 2 3 4 Carter EL, Jager L, Gardner L, Hall CC, Willis S, Green JM (May 2007). "Escherichia coli abg genes enable uptake and cleavage of the folate catabolite p-aminobenzoyl-glutamate". Journal of Bacteriology. 189 (9): 3329–34. doi:10.1128/JB.01940-06. PMC 1855889 . PMID 17307853.
- ↑ Su CC, Bolla JR, Kumar N, Radhakrishnan A, Long F, Delmar JA, Chou TH, Rajashankar KR, Shafer WM, Yu EW (April 2015). "Structure and function of Neisseria gonorrhoeae MtrF illuminates a class of antimetabolite efflux pumps". Cell Reports. 11 (1): 61–70. doi:10.1016/j.celrep.2015.03.003. PMC 4410016 . PMID 25818299.
- 1 2 3 Delmar JA, Yu EW (February 2016). "The AbgT family: A novel class of antimetabolite transporters". Protein Science. 25 (2): 322–37. doi:10.1002/pro.2820. PMC 4815354 . PMID 26443496.
- ↑ Bolla JR, Su CC, Delmar JA, Radhakrishnan A, Kumar N, Chou TH, Long F, Rajashankar KR, Yu EW (April 2015). "Crystal structure of the Alcanivorax borkumensis YdaH transporter reveals an unusual topology". Nature Communications. 6: 6874. Bibcode:2015NatCo...6.6874B. doi:10.1038/ncomms7874. PMC 4410182 . PMID 25892120.