Related Research Articles
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originate from food, chemicals, pharmaceuticals, biological, environmental and agriculture, etc., which have been dissolved into liquid solutions.

Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.

Archer John Porter Martin was a British chemist who shared the 1952 Nobel Prize in Chemistry for the invention of partition chromatography with Richard Synge.

Paper chromatography is an analytical method used to separate colored chemicals or substances. It can also be used for colorless chemicals that can be located by a stain or other visualisation method after separation. It is now primarily used as a teaching tool, having been replaced in the laboratory by other chromatography methods such as thin-layer chromatography (TLC).

Column chromatography in chemistry is a chromatography method used to isolate a single chemical compound from a mixture. Chromatography is able to separate substances based on differential absorption of compounds to the adsorbent; compounds move through the column at different rates, allowing them to be separated into fractions. The technique is widely applicable, as many different adsorbents can be used with a wide range of solvents. The technique can be used on scales from micrograms up to kilograms. The main advantage of column chromatography is the relatively low cost and disposability of the stationary phase used in the process. The latter prevents cross-contamination and stationary phase degradation due to recycling. Column chromatography can be done using gravity to move the solvent, or using compressed gas to push the solvent through the column.

Thin-layer chromatography (TLC) is a chromatography technique that separates components in non-volatile mixtures.

Solid-phase extraction (SPE) is a solid-liquid extractive technique, by which compounds that are dissolved or suspended in a liquid mixture are separated, isolated or purified, from other compounds in this mixture, according to their physical and chemical properties. Analytical laboratories use solid phase extraction to concentrate and purify samples for analysis. Solid phase extraction can be used to isolate analytes of interest from a wide variety of matrices, including urine, blood, water, beverages, soil, and animal tissue.
Chiral column chromatography is a variant of column chromatography that is employed for the separation of chiral compounds, i.e. enantiomers, in mixtures such as racemates or related compounds. The chiral stationary phase (CSP) is made of a support, usually silica based, on which a chiral reagent or a macromolecule with numerous chiral centers is bonded or immobilized.
Countercurrent distribution is an analytical chemistry technique which was developed by Lyman C. Craig in the 1940s. Countercurrent distribution is a separation process that is founded on the principles of liquid–liquid extraction where a chemical compound is distributed (partitioned) between two immiscible liquid phases according to its relative solubility in the two phases. The simplest form of liquid-liquid extraction is the partitioning of a mixture of compounds between two immiscible liquid phases in a separatory funnel. This occurs in five steps: 1) preparation of the separatory funnel with the two phase solvent system, 2) introduction of the compound mixture into the separatory funnel, 3) vigorous shaking of the separatory funnel to mix the two layers and allow for mass transfer of compounds in and out of the phases, 4) The contents of the separatory funnel are allowed to settle back into two distinct phases and 5) the two phases are separated from each other by draining out the bottom phase. If a compound is insoluble in the lower phase it will distribute into the upper phase and stay in the separatory funnel. If a compound is insoluble in the upper phase it will distribute into the lower phase and be removed from the separatory funnel. If the mixture contains one or more compounds that are soluble in the upper phase and one or more compounds that are soluble in the lower phase, then an extraction has occurred. Often, an individual compound is soluble to a certain extent in both phases and the extraction is, therefore, incomplete. The relative solubility of a compound in two phases is known as the partition coefficient.
Reversed-phase liquid chromatography (RP-LC) is a mode of liquid chromatography in which non-polar stationary phase and polar mobile phases are used for the separation of organic compounds. The vast majority of separations and analyses using high-performance liquid chromatography (HPLC) in recent years are done using the reversed phase mode. In the reversed phase mode, the sample components are retained in the system the more hydrophobic they are.
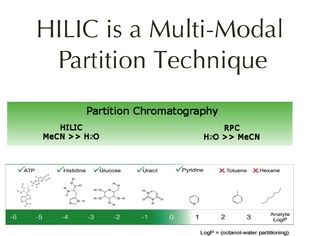
Hydrophilic interaction chromatography is a variant of normal phase liquid chromatography that partly overlaps with other chromatographic applications such as ion chromatography and reversed phase liquid chromatography. HILIC uses hydrophilic stationary phases with reversed-phase type eluents. The name was suggested by Andrew Alpert in his 1990 paper on the subject. He described the chromatographic mechanism for it as liquid-liquid partition chromatography where analytes elute in order of increasing polarity, a conclusion supported by a review and re-evaluation of published data.
Aqueous normal-phase chromatography (ANP) is a chromatographic technique that involves the mobile phase compositions and polarities between reversed-phase chromatography (RP) and normal-phase chromatography (NP), while the stationary phases are polar.
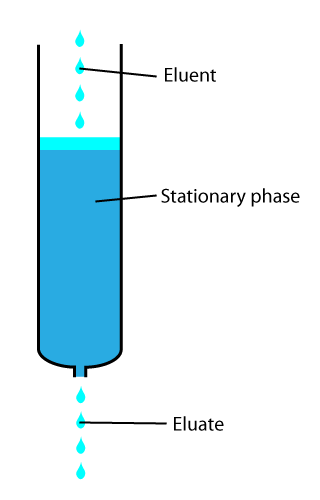
In analytical and organic chemistry, elution is the process of extracting one material from another by washing with a solvent: washing of loaded ion-exchange resins to remove captured ions, or eluting proteins or other biopolymers from a gel electrophoresis or chromatography column.
The history of chromatography spans from the mid-19th century to the 21st. Chromatography, literally "color writing", was used—and named— in the first decade of the 20th century, primarily for the separation of plant pigments such as chlorophyll and carotenoids. New forms of chromatography developed in the 1930s and 1940s made the technique useful for a wide range of separation processes and chemical analysis tasks, especially in biochemistry.

Erika Cremer was a German physical chemist and Professor Emeritus at the University of Innsbruck who is regarded as one of the most important pioneers in gas chromatography, as she second conceived the technique in 1944, after Richard Synge and Archer J.P. Martin in 1941.
The Wool Industries Research Association was an industrial research organization in the United Kingdom. It later became the Wira Technology Group before being merged with the Shirley Institute in the 1989 to form the British Textile Technology Group. It was funded by a levy raised under powers from the Industrial Organisation and Development Act 1947 through the Wool Textile Research Council, established in 1950.

Countercurrent chromatography is a form of liquid–liquid chromatography that uses a liquid stationary phase that is held in place by inertia of the molecules composing the stationary phase accelerating toward the center of a centrifuge due to centripetal force and is used to separate, identify, and quantify the chemical components of a mixture. In its broadest sense, countercurrent chromatography encompasses a collection of related liquid chromatography techniques that employ two immiscible liquid phases without a solid support. The two liquid phases come in contact with each other as at least one phase is pumped through a column, a hollow tube or a series of chambers connected with channels, which contains both phases. The resulting dynamic mixing and settling action allows the components to be separated by their respective solubilities in the two phases. A wide variety of two-phase solvent systems consisting of at least two immiscible liquids may be employed to provide the proper selectivity for the desired separation.
Droplet countercurrent chromatography was introduced in 1970 by Tanimura, Pisano, Ito, and Bowman. DCCC is considered to be a form of liquid-liquid separation, which includes countercurrent distribution and countercurrent chromatography, that employs a liquid stationary phase held in a collection of vertical glass columns connected in series. The mobile phase passes through the columns in the form of droplets. The DCCC apparatus may be run with the lower phase stationary and the upper phase being introduced to the bottom of each column. Or it may be run with the upper phase stationary and the lower phase being introduced from the top of the column. In both cases, the work of gravity is allowed influence the two immiscible liquids of different densities to form the signature droplets that rise or descend through the column. The mobile phase is pumped at a rate that will allow droplets to form that maximize the mass transfer of a compound between the upper and lower phases. Compounds that are more soluble in the upper phase will travel quickly through the column, while compounds that are more soluble in the stationary phase will linger. Separation occurs because different compounds distribute differently, in a ratio called the partition coefficient, between the two phases.
Chiral analysis refers to the quantification of component enantiomers of racemic drug substances or pharmaceutical compounds. Other synonyms commonly used include enantiomer analysis, enantiomeric analysis, and enantioselective analysis. Chiral analysis includes all analytical procedures focused on the characterization of the properties of chiral drugs. Chiral analysis is usually performed with chiral separation methods where the enantiomers are separated on an analytical scale and simultaneously assayed for each enantiomer.
References
- 1 2 Ettre, Leslie S. (2001). "The Birth of Partition Chromatography". LCGC. 19 (5): 506–512. INIST 990422.
- ↑ "The Nobel Prize in Chemistry 1952". Nobel Media AB. Retrieved 4 February 2021.
- ↑ Pakhomov, V. P. (2003). "Chromatography in Pharmaceutical Chemistry (100 Years of the Discovery of Chromatography by M. S. Tswett)". Pharmaceutical Chemistry Journal. 37 (8): 451–452. doi:10.1023/A:1027324501053. S2CID 22507057.
- ↑ Schindler, Hans (November 1957). "Notes on the history of the separatory funnel". Journal of Chemical Education. 34 (11): 528. Bibcode:1957JChEd..34..528S. doi:10.1021/ed034p528.
- ↑ Martin, A. J. P.; Synge, R. L. M. (1 January 1941). "Separation of the higher monoamino-acids by counter-current liquid-liquid extraction: the amino-acid composition of wool". Biochemical Journal. 35 (1–2): 91–121. doi:10.1042/bj0350091. PMC 1265473 . PMID 16747393.
- ↑ Randall, Merle; Longtin, Bruce (September 1938). "Separation Processes: General Method of Analysis". Industrial & Engineering Chemistry. 30 (9): 1063–1067. doi:10.1021/ie50345a028.
- ↑ Whelan, William J. (1 May 2001). "Partition Chromatography Revisited". IUBMB Life. 51 (5): 329–330. doi: 10.1080/152165401317190851 . PMID 11699880.
- ↑ Martin, A. J. P.; Synge, R. L. M. (1 December 1941). "A new form of chromatogram employing two liquid phases. A theory of chromatography. 2. Application to the micro-determination of the higher monoamino-acids in proteins". Biochemical Journal. 35 (12): 1358–1368. doi:10.1042/bj0351358. PMC 1265645 . PMID 16747422.
- ↑ Craig, Lyman C. (October 1944). "Identification of small amounts of organic compounds by distribution studies: II. Separation by counter-current distribution". Journal of Biological Chemistry. 155 (2): 519–534. doi: 10.1016/S0021-9258(18)51183-2 .
- ↑ Consden, R.; Gordon, A. H.; Martin, A. J. P. (1944). "Qualitative analysis of proteins: A partition chromatographic method using paper". Biochemical Journal. 38 (3): 224–232. doi:10.1042/bj0380224. PMC 1258072 . PMID 16747784.
- ↑ Horvath, C.; Melander, W. (1 September 1977). "Liquid Chromatography with Hydrocarbonaceous Bonded Phases; Theory and Practice of Reversed Phase Chromatography". Journal of Chromatographic Science. 15 (9): 393–404. doi:10.1093/chromsci/15.9.393.
- ↑ Moore, Stanford (1978). "Lyman Creighton Craig 1906-1974". National Academy of Sciences Biographical Memoirs: 49–77. Retrieved 2016-02-26.
- ↑ Martin, A J P (June 1950). "Partition Chromatography". Annual Review of Biochemistry. 19 (1): 517–542. doi:10.1146/annurev.bi.19.070150.002505. PMID 14771840.
- ↑ James, A. T.; Martin, A. J. P.; Smith, G. Howard (1 October 1952). "Gas-liquid partition chromatography: the separation and micro-estimation of ammonia and the methylamines". Biochemical Journal. 52 (2): 238–242. doi:10.1042/bj0520238. PMC 1197975 . PMID 13018213.