Related Research Articles

The retina is the innermost, light-sensitive layer of tissue of the eye of most vertebrates and some molluscs. The optics of the eye create a focused two-dimensional image of the visual world on the retina, which then processes that image within the retina and sends nerve impulses along the optic nerve to the visual cortex to create visual perception. The retina serves a function which is in many ways analogous to that of the film or image sensor in a camera.
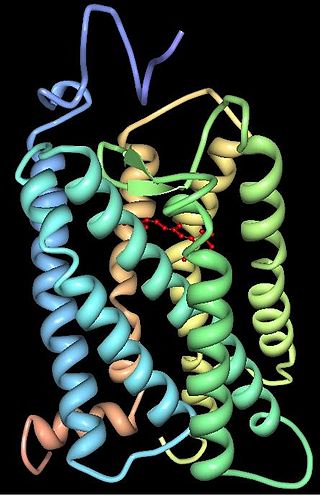
Rhodopsin, also known as visual purple, is a protein encoded by the RHO gene and a G-protein-coupled receptor (GPCR). It is the opsin of the rod cells in the retina and a light-sensitive receptor protein that triggers visual phototransduction in rods. Rhodopsin mediates dim light vision and thus is extremely sensitive to light. When rhodopsin is exposed to light, it immediately photobleaches. In humans, it is regenerated fully in about 30 minutes, after which the rods are more sensitive. Defects in the rhodopsin gene cause eye diseases such as retinitis pigmentosa and congenital stationary night blindness.

The visual system is the physiological basis of visual perception. The system detects, transduces and interprets information concerning light within the visible range to construct an image and build a mental model of the surrounding environment. The visual system is associated with the eye and functionally divided into the optical system and the neural system.

Retinitis pigmentosa (RP) is a genetic disorder of the eyes that causes loss of vision. Symptoms include trouble seeing at night and decreasing peripheral vision. As peripheral vision worsens, people may experience "tunnel vision". Complete blindness is uncommon. Onset of symptoms is generally gradual and often begins in childhood.
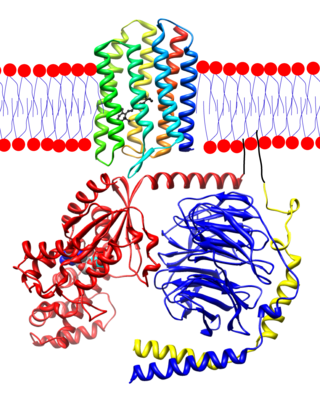
Transducin (Gt) is a protein naturally expressed in vertebrate retina rods and cones and it is very important in vertebrate phototransduction. It is a type of heterotrimeric G-protein with different α subunits in rod and cone photoreceptors.
In visual physiology, adaptation is the ability of the retina of the eye to adjust to various levels of light. Natural night vision, or scotopic vision, is the ability to see under low-light conditions. In humans, rod cells are exclusively responsible for night vision as cone cells are only able to function at higher illumination levels. Night vision is of lower quality than day vision because it is limited in resolution and colors cannot be discerned; only shades of gray are seen. In order for humans to transition from day to night vision they must undergo a dark adaptation period of up to two hours in which each eye adjusts from a high to a low luminescence "setting", increasing sensitivity hugely, by many orders of magnitude. This adaptation period is different between rod and cone cells and results from the regeneration of photopigments to increase retinal sensitivity. Light adaptation, in contrast, works very quickly, within seconds.
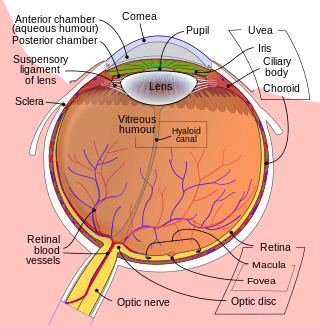
The fovea centralis is a small, central pit composed of closely packed cones in the eye. It is located in the center of the macula lutea of the retina.
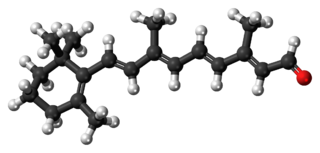
Retinal is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).

Melanopsin is a type of photopigment belonging to a larger family of light-sensitive retinal proteins called opsins and encoded by the gene Opn4. In the mammalian retina, there are two additional categories of opsins, both involved in the formation of visual images: rhodopsin and photopsin in the rod and cone photoreceptor cells, respectively.

Animal opsins are G-protein-coupled receptors and a group of proteins made light-sensitive via a chromophore, typically retinal. When bound to retinal, opsins become retinylidene proteins, but are usually still called opsins regardless. Most prominently, they are found in photoreceptor cells of the retina. Five classical groups of opsins are involved in vision, mediating the conversion of a photon of light into an electrochemical signal, the first step in the visual transduction cascade. Another opsin found in the mammalian retina, melanopsin, is involved in circadian rhythms and pupillary reflex but not in vision. Humans have in total nine opsins. Beside vision and light perception, opsins may also sense temperature, sound, or chemicals.
The Stiles–Crawford effect is a property of the human eye that refers to the directional sensitivity of the cone photoreceptors.
Rhodopsin kinase is a serine/threonine-specific protein kinase involved in phototransduction. This enzyme catalyses the following chemical reaction:

Blue-sensitive opsin is a protein that in humans is encoded by the OPN1SW gene.

Green-sensitive opsin is a protein that in humans is encoded by the OPN1MW gene. OPN1MW2 is a similar opsin.

Guanylyl cyclase-activating protein 2 is an enzyme that in humans is encoded by the GUCA1B gene. Alternative names:
Krzysztof Palczewski is a Polish-American biochemist working at the University of California, Irvine.

Retinal degeneration is a retinopathy which consists in the deterioration of the retina caused by the progressive death of its cells. There are several reasons for retinal degeneration, including artery or vein occlusion, diabetic retinopathy, R.L.F./R.O.P., or disease. These may present in many different ways such as impaired vision, night blindness, retinal detachment, light sensitivity, tunnel vision, and loss of peripheral vision to total loss of vision. Of the retinal degenerative diseases retinitis pigmentosa (RP) is a very important example.
Aristostomias is a genus of barbeled dragonfishes native to the ocean depths in the Pacific, Atlantic and Indian oceans.

King-Wai Yau is a Chinese-born American neuroscientist and Professor of Neuroscience at Johns Hopkins University School of Medicine in Baltimore, Maryland.

Davida Young Teller was a professor in the Departments of Psychology and Physiology/Biophysics at the University of Washington, Seattle, Washington. She was a leader in the scientific study of infant visual development.
References
- ↑ American Men and Women of Science: Chemistry. Bowker. 1977. p. 479. ISBN 9780835210119 . Retrieved 26 November 2018.
- ↑ American men & women of science: Physical and Biological Sciences. Bowker. 1989. p. 501. ISBN 9780835214179 . Retrieved 26 November 2018.
- ↑ Papermaster, David S. (2001-01-01). "Introducing Paul Hargrave, the 2000 Recipient of the Friedenwald Award". Investigative Ophthalmology & Visual Science. 42 (1): 1–2. ISSN 1552-5783. PMID 11133840.
- ↑ Hargrave, P. A. (1977-05-27). "The amino-terminal tryptic peptide of bovine rhodopsin. A glycopeptide containing two sites of oligosaccharide attachment". Biochimica et Biophysica Acta (BBA) - Protein Structure. 492 (1): 83–94. doi:10.1016/0005-2795(77)90216-1. ISSN 0006-3002. PMID 861254.
- ↑ Hargrave, Paul A (1982-01-01). "The carboxyl-terminal one-third of bovine rhodopsin: Its structure and function". Vision Research. 22 (12). et al: 1429–1438. doi:10.1016/0042-6989(82)90205-X. ISSN 0042-6989. PMID 7182998. S2CID 1556264.
- ↑ Hargrave, P. A.; McDowell, J. H.; Curtis, Donna R.; Wang, Janet K.; Juszczak, Elizabeth; Fong, Shao-Ling; Mohana Rao, J. K.; Argos, P. (1983). "The structure of bovine rhodopsin". Biophysics of Structure and Mechanism. 9 (4): 235–244. doi:10.1007/bf00535659. ISSN 0340-1057. PMID 6342691. S2CID 20407577.
- ↑ Hargrave, P. A.; McDowell, J. H.; Feldmann, R. J.; Atkinson, P. H.; Rao, J. K.; Argos, P. (1984). "Rhodopsin's protein and carbohydrate structure: selected aspects". Vision Research. 24 (11): 1487–1499. doi:10.1016/0042-6989(84)90311-0. ISSN 0042-6989. PMID 6533983. S2CID 31984423.
- ↑ Dratz, Edward; Hargrave, Paul A (1983-04-01). "The structure of rhodopsin and the rod outer segment disk membrane". Trends in Biochemical Sciences. 8 (4): 128–131. doi:10.1016/0968-0004(83)90235-9. ISSN 0968-0004.
- ↑ Palczewski, Krzysztof (February 2011). "Focus on vision: 3 decades of remarkable contributions to biology and medicine". The FASEB Journal. 25 (2): 439–443. doi: 10.1096/fj.11-0202ufm . ISSN 0892-6638. PMC 3228347 . PMID 21282210.
- ↑ Papermaster, David S. (2001-01-01). "Introducing Paul Hargrave, the 2000 Recipient of the Friedenwald Award". Investigative Ophthalmology & Visual Science. 42 (1): 1–2. ISSN 1552-5783. PMID 11133840.
- ↑ "News and Comment". Archives of Ophthalmology. 103 (4): 492. 1985-04-01. doi:10.1001/archopht.1985.01050040034012. ISSN 0003-9950.
- ↑ "Paul Hargrave, Ph.D. | UFRF Professors". ufrfprofessors.feed.research.ufl.edu. Retrieved 2018-11-16.
- ↑ Unger, Vinzenz M.; Hargrave, Paul A.; Baldwin, Joyce M.; Schertler, Gebhard F. X. (September 1997). "Arrangement of rhodopsin transmembrane α-helices". Nature. 389 (6647): 203–206. Bibcode:1997Natur.389..203U. doi:10.1038/38316. ISSN 0028-0836. PMID 9296501. S2CID 205026444.
- ↑ Hargrave, Paul A; McDowell, J Hugh (1993-01-01). "Rhodopsin and Phototransduction". International Review of Cytology. 137: 49–97. doi:10.1016/S0074-7696(08)62600-5. ISBN 9780123645388. ISSN 0074-7696. PMID 1478822.
- ↑ Deretic, Dusanka; Williams, Andrew H.; Ransom, Nancy; Morel, Valerie; Hargrave, Paul A.; Arendt, Anatol (2005-03-01). "Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4)". Proceedings of the National Academy of Sciences. 102 (9): 3301–3306. Bibcode:2005PNAS..102.3301D. doi: 10.1073/pnas.0500095102 . ISSN 0027-8424. PMC 552909 . PMID 15728366.
- ↑ Vrabec, Tamara; Arbizo, Violeta; Adamus, Grazyna; McDowell, J Hugh; Hargrave, PA; Donoso, Larry (1989-07-01). "Rod Cell-Specific Antigens in Retinoblastoma". Archives of Ophthalmology. 107 (7): 1061–3. doi:10.1001/archopht.1989.01070020123044. ISSN 0003-9950. PMID 2473730.
- ↑ Kefalov, Vladimir J.; Arshavsky, Vadim Y. (June 2014). "FASEB Science Research Conference on Biology and Chemistry of Vision". The FASEB Journal. 28 (6): 2395–2397. doi: 10.1096/fj.14-0602ufm . ISSN 0892-6638. PMC 6137602 . PMID 24891609.
- ↑ Paul, Hargrave. "FASEB Conference: Biology and Chemistry of Vision August 11-16, 1985; Saxton's River, Vermont". Grantome.
- ↑ Papermaster, David S. (2001-01-01). "Introducing Paul Hargrave, the 2000 Recipient of the Friedenwald Award". Investigative Ophthalmology & Visual Science. 42 (1): 1–2. ISSN 1552-5783. PMID 11133840.
- ↑ "International society honors UF vision scientist". UF Health, University of Florida Health. 2000-06-07. Retrieved 2018-11-17.
- ↑ "USA Male Finishers by Date". 50 States Club. Retrieved 2018-11-16.
- ↑ @elizhargrave (October 13, 2020). "(Elizabeth Hargrave links to this Wikipedia profile as belonging to her father)" (Tweet) – via Twitter.