
Coffinite is a uranium-bearing silicate mineral with formula: U(SiO4)1−x(OH)4x.

Yellowcake is a type of powdered uranium concentrate obtained from leach solutions, in an intermediate step in the processing of uranium ores. It is a step in the processing of uranium after it has been mined but before fuel fabrication or uranium enrichment. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores. Typically, yellowcakes are obtained through the milling and chemical processing of uranium ore, forming a coarse powder that has a pungent odor, is insoluble in water, and contains about 80% uranium oxide, which melts at approximately 2880 °C.

Torbernite, also known as chalcolite, is a relatively common mineral with the chemical formula Cu[(UO2)(PO4)]2(H2O)12. It is a radioactive, hydrated green copper uranyl phosphate, found in granites and other uranium-bearing deposits as a secondary mineral. The chemical formula of torbernite is similar to that of autunite in which a Cu2+ cation replaces a Ca2+ cation. Torbernite tends to dehydrate to metatorbernite with the sum formula Cu[(UO2)(PO4)]2(H2O)8.

Zippeite is a hydrous potassium uranium sulfate mineral with formula: K4(UO2)6(SO4)3(OH)10·4(H2O). It forms yellow to reddish brown monoclinic-prismatic crystals with perfect cleavage. The typical form is as encrustations and pulverulent earthy masses. It forms as efflorescent encrustations in underground uranium mines. It has a Mohs hardness of 2 and a specific gravity of 3.66. It is strongly fluorescent yellow under ultraviolet light and is moderately radioactive.

Uranium oxide is an oxide of the element uranium.

Uranium trioxide (UO3), also called uranyl oxide, uranium(VI) oxide, and uranic oxide, is the hexavalent oxide of uranium. The solid may be obtained by heating uranyl nitrate to 400 °C. Its most commonly encountered polymorph is amorphous UO3.

Uranyl hydroxide is a hydroxide of uranium with the chemical formula UO2(OH)2 in the monomeric form and [(UO2)2(OH)4]2- in the dimeric; both forms may exist in normal aqueous media. In aerobic conditions, up to 5 hydroxides can bind to Uranyl {[(UO2)2(OH)5]3-}. Uranyl hydroxide hydrate is precipitated as a colloidal yellowcake from oxidized uranium liquors near neutral pH.

Uranyl peroxide or uranium peroxide hydrate (UO4·nH2O) is a pale-yellow, soluble peroxide of uranium. It is found to be present at one stage of the enriched uranium fuel cycle and in yellowcake prepared via the in situ leaching and resin ion exchange system. This compound, also expressed as UO3·(H2O2)·(H2O), is very similar to uranium trioxide hydrate UO3·nH2O. The dissolution behaviour of both compounds are very sensitive to the hydration state (n can vary between 0 and 4). One main characteristic of uranium peroxide is that it consists of small needles with an average AMAD of about 1.1 μm.

Ammonium diuranate or (ADU) ((NH4)2U2O7), is one of the intermediate chemical forms of uranium produced during yellowcake production. The name "yellowcake" originally given to this bright yellow salt, now applies to mixtures of uranium oxides which are actually hardly ever yellow. It also is an intermediate in mixed-oxide (MOX) fuel fabrication. Although it is usually called "ammonium diuranate" as though it has a "diuranate" ion U
2O2−
7, this is not necessarily the case. It can also be called diammonium diuranium heptaoxide. The structure was theorized to be similar to that of uranium trioxide dihydrate. Recent literature has shown that the structure more closely resembles the mineral metaschoepite, the partially dehydrated form of schoepite.

Schoepite, empirical formula (UO2)8O2(OH)12·12(H2O) is a rare alteration product of uraninite in hydrothermal uranium deposits. It may also form directly from ianthinite. The mineral presents as a transparent to translucent yellow, lemon yellow, brownish yellow, or amber orthorhombic tabular crystals. Although over 20 other crystal forms have been noted; rarely in microcrystalline aggregates. When exposed to air schoepite converts over a short time to the metaschoepite form (UO3·nH2O, n < 2) within a few months of being exposed to ambient air.

Bergenite is a rare uranyl phosphate of the more specific phosphuranylite group. The phosphuranylite-type sheet in bergenite is a new isomer of the group, with the uranyl phosphate tetrahedra varying in an up-up-down, same-same-opposite (uuduudSSOSSO) orientation. All bergenite samples have been found in old mine dump sites. Uranyl minerals are a large constituent of uranium deposits.

Boltwoodite is a hydrated uranyl silicate mineral with formula (K0.56Na0.42)[(UO2)(SiO3OH)]·1.5(H2O), distinct in crystal structure from sodium boltwoodite, which has an orthorhombic structure rather than monoclinic. It is formed from the oxidation and alteration of primary uranium ores. It takes the form of a crust on some sandstones that bear uranium. These crusts tend to be yellowish with a silky or vitreous luster.

Curite is a rare mineral with the chemical composition Pb3[(UO2)4|O4|(OH)3]2·2 H2O. It is therefore a hydrated lead uranyl oxide, which forms red needles or orange, massive aggregates.
Bijvoetite-(Y) is a very rare rare-earth and uranium mineral with the formula (Y,REE)8(UO2)16(CO3)16O8(OH)8·39H2O. When compared to the original description, the formula of bijvoetite-(Y) was changed in the course of crystal structure redefinition. Bijvoetite-(Y) is an example of natural salts containing both uranium and yttrium, the other examples being kamotoite-(Y) and sejkoraite-(Y). Bijvoetite-(Y) comes from Shinkolobwe deposit in Republic of Congo, which is famous for rare uranium minerals. The other interesting rare-earth-bearing uranium mineral, associated with bijvoetite-(Y), is lepersonnite-(Gd).
Meisserite is a very rare uranium mineral with the formula Na5(UO2)(SO4)3(SO3OH)(H2O). It is interesting in being a natural uranyl salt with hydrosulfate (hydroxysulfate) anion, a feature shared with belakovskiite. Other chemically related minerals include fermiite, oppenheimerite, natrozippeite and plášilite. Most of these uranyl sulfate minerals was originally found in the Blue Lizard mine, San Juan County, Utah, USA. The mineral is named after Swiss mineralogist Nicolas Meisser.
Meyrowitzite, Ca(UO2)(CO3)2·5H2O, is a carbonate mineral verified in May of 2018 by the Commission of New Minerals, Nomenclature and Classification of the International Mineralogical Association. It is an extremely rare mineral, discovered in the Markey mine Utah, U.S.A. The mineral is a transparent yellow and has blades up to approximately 0.2 mm in length. It is soluble in water or aqueous solutions. Meyrowitzite is named in honor of Robert Meyrowitz (1916–2013), an American analytical chemist. After serving in WW II, he joined the United States Geological Survey (USGS). He was known for developing innovative new methods for analyzing small and difficult to study mineralogical samples along with his formulation of the high-index immersion liquids.
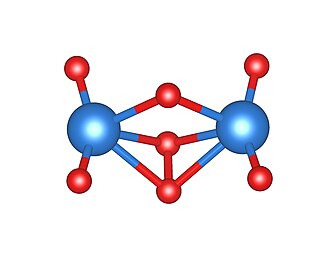
Amorphous uranium(VI) oxide(am-U2O7) is an orange diuranyl compound, most commonly obtained from the thermal decomposition of uranyl peroxide tetrahydrate at temperatures between 150 and 500 °C (300 and 930 °F). It exists at room temperature as a powder. Am-U2O7 does not comprise a regular, long-range atomic structure, as demonstrated by its characteristic diffuse scattering pattern obtained by X-ray diffraction. As a result, the molecular structure of this material is little understood, although experimental and computational attempts to elucidate a local atomic environment have yielded some success.

Zellerite is a uranium mineral, named after its discoverer, geologist Howard Davis Zeller. It has a type locality of the Lucky MC uranium mine in Wyoming, USA. It was approved by the IMA in 1965, but was first published a year after its approval.

Gauthierite is a very rare mineral with the idealised chemical sum formula KPb[(UO2)7O5(OH)7]·8H2O. It is a radioactive, hydrated orange-coloured lead potassium uranyl oxide hydroxide. It was found by analysing old mineral specimens, and is only known from one locality, the Shinkolobwe Mine in the Democratic Republic of the Congo. The mineral was named in honour of Gilbert Gauthier, a Belgian collector of uranium minerals, who provided a sample to one of the co-authors of the study that first identified it in 2017.
Uramphite is a rarely-found phosphate mineral in the "phosphate, arsenate and vanadate" mineral class with chemical composition (NH4)2[UO2PO4]2·6H2O from which it is seen to be a hydrated ammonium uranyl phosphate.














