In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.

Bioequivalence is a term in pharmacokinetics used to assess the expected in vivo biological equivalence of two proprietary preparations of a drug. If two products are said to be bioequivalent it means that they would be expected to be, for all intents and purposes, the same.
Fulvestrant, sold under the brand name Faslodex among others, is an antiestrogenic medication used to treat hormone receptor (HR)-positive metastatic breast cancer in postmenopausal women with disease progression as well as HR-positive, HER2-negative advanced breast cancer in combination with abemaciclib or palbociclib in women with disease progression after endocrine therapy. It is given by injection into a muscle.

Piperacillin is a broad-spectrum β-lactam antibiotic of the ureidopenicillin class. The chemical structure of piperacillin and other ureidopenicillins incorporates a polar side chain that enhances penetration into Gram-negative bacteria and reduces susceptibility to cleavage by Gram-negative beta lactamase enzymes. These properties confer activity against the important hospital pathogen Pseudomonas aeruginosa. Thus piperacillin is sometimes referred to as an "anti-pseudomonal penicillin".
In medicine and pharmacology, a trough level or trough concentration is the concentration reached by a drug immediately before the next dose is administered, often used in therapeutic drug monitoring. The name comes from the idea that on a graph of concentration versus time, the line forms a U-shaped trough at the lowest region, before a new dose sends it higher again. The usual criterion is concentration in the blood serum, although in some instances local concentration within tissues is relevant. It is pharmacokinetically normal that over time, the drug molecules are being metabolized or cleared by the body, so the concentration of drug that remains available is dropping. In a medicine that is administered periodically, the trough level should be measured just before the administration of the next dose in order to avoid overdosing. A trough level is contrasted with a "peak level", which is the highest level of the medicine in the body, and the "average level", which is the mean level over time. It is widely used in clinical trials for newer medicines to investigate therapeutic effectiveness and safety.

Levmetamfetamine, also known as l-desoxyephedrine or levomethamphetamine, and commonly sold under the brand name Vicks VapoInhaler among others, is an optical isomer of methamphetamine primarily used as a topical nasal decongestant. It is used to treat nasal congestion from allergies and the common cold. It was first used medically as decongestant beginning in 1958 and has been used for such purposes, primarily in the United States, since then.
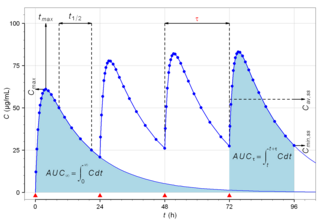
Biological half-life is the time taken for concentration of a biological substance to decrease from its maximum concentration (Cmax) to half of Cmax in the blood plasma. It is denoted by the abbreviation .

Acebutolol, sold under the brand names Sectral among others, is a beta blocker for the treatment of hypertension and arrhythmias. Acebutolol is a cardioselective beta-1 blocker and has intrinsic sympathetic activity. It is commonly used in the treatment of angina.

Phenprocoumon is a long-acting blood thinner drug to be taken by mouth, and a coumarin derivative. It acts as a vitamin K antagonist and inhibits blood clotting (coagulation) by blocking synthesis of coagulation factors II, VII, IX and X. It is used for the prophylaxis and treatment of thromboembolic disorders such as heart attacks and pulmonary (lung) embolism. The most common adverse effect is bleeding. The drug interacts with a large number of other medications, including aspirin and St John's Wort. It is the standard coumarin used in Germany, Austria, and other European countries.

Acecainide is an antiarrhythmic drug. Chemically, it is the N-acetylated metabolite of procainamide. It is a Class III antiarrhythmic agent, whereas procainamide is a Class Ia antiarrhythmic drug. It is only partially as active as procainamide; when checking levels, both must be included in the final calculation.

Sparfloxacin is a fluoroquinolone antibiotic used in the treatment of bacterial infections. It has a controversial safety profile.

Pharmacokinetics, sometimes abbreviated as PK, is a branch of pharmacology dedicated to describing how the body affects a specific substance after administration. The substances of interest include any chemical xenobiotic such as pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is based on mathematical modeling that places great emphasis on the relationship between drug plasma concentration and the time elapsed since the drug's administration. Pharmacokinetics is the study of how an organism affects the drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effects, as seen in PK/PD models.
Cmin is a term used in pharmacokinetics for the minimum blood plasma concentration reached by a drug during a dosing interval, which is the time interval between administration of two doses. This definition is slightly different from Ctrough, the concentration immediately prior to administration of the next dose. Cmin is the opposite of Cmax, the maximum concentration that the drug reaches. Cmin must be above certain thresholds, such as the minimum inhibitory concentration (MIC), to achieve a therapeutic effect.
Cmax is the maximum serum concentration that a drug achieves in a specified compartment or test area of the body after the drug has been administered and before the administration of a second dose. It is a standard measurement in pharmacokinetics.
Bilastine is an antihistamine medication used to treat hives (urticaria), allergic rhinitis and itchy inflamed eyes (allergic conjunctivitis) caused by an allergy. It is a second-generation antihistamine and takes effect by selectively inhibiting the histamine H1 receptor, preventing these allergic reactions. Bilastine has an effectiveness similar to cetirizine, fexofenadine, and desloratadine.

Oxogestone phenpropionate, also known as xinogestone, as well as 20β-hydroxy-19-norprogesterone 20β-(3-phenylpropionate), is a progestin related to the 19-norprogesterone derivatives which was developed as an injectable hormonal contraceptive, specifically a progestogen-only injectable contraceptive, in the 1960s and early 1970s but was never marketed. It was studied at a dose of 50 to 75 mg once a month by intramuscular injection but was associated with a high failure rate with this regimen and was not further developed. OPP is the 20β-(3-phenylpropionate) ester of oxogestone, which, similarly, was never marketed.

Nordoxepin, also known as N-desmethyldoxepin, is an organic compound. A colorless solid, it attracted attention as the major active metabolite of the tricyclic antidepressant (TCA) doxepin (Sinequan). It has been found to play a significant role in the antidepressant effects of doxepin.

The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.
In pharmacokinetics, the drug accumulation ratio (Rac) is the ratio of accumulation of a drug under steady state conditions as compared to a single dose. The higher the value, the more the drug accumulates in the body. An Rac of 1 means no accumulation.

Lynestrenol phenylpropionate (LPP), also known as ethynylestrenol phenylpropionate, is a progestin and a progestogen ester which was developed for potential use as a progestogen-only injectable contraceptive by Organon but was never marketed. It was assessed at doses of 25 to 75 mg in an oil solution once a month by intramuscular injection. LPP was associated with high contraceptive failure at the low dose and with poor cycle control. The medication was found to produce estrogenic effects in the endometrium in women due to transformation into estrogenic metabolites.













