Related Research Articles

Irinotecan, sold under the brand name Camptosar among others, is a medication used to treat colon cancer, and small cell lung cancer. For colon cancer it is used either alone or with fluorouracil. For small cell lung cancer it is used with cisplatin. It is given by slow injection into a vein.
The nomenclature of monoclonal antibodies is a naming scheme for assigning generic, or nonproprietary, names to monoclonal antibodies. An antibody is a protein that is produced in B cells and used by the immune system of humans and other vertebrate animals to identify a specific foreign object like a bacterium or a virus. Monoclonal antibodies are those that were produced in identical cells, often artificially, and so share the same target object. They have a wide range of applications including medical uses.

Certolizumab pegol, sold under the brand name Cimzia, is a biologic medication for the treatment of Crohn's disease, rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. It is a fragment of a monoclonal antibody specific to tumor necrosis factor alpha (TNF-α) and is manufactured by UCB.

Pegaspargase, sold under the brand name Oncaspar, is a medication used in the treatment of acute lymphoblastic leukemia (ALL). Often it is used together with anthracycline, vincristine, and prednisone. It is used by injection.

Biological therapy, the use of medications called biopharmaceuticals or biologics that are tailored to specifically target an immune or genetic mediator of disease, plays a major role in the treatment of inflammatory bowel disease. Even for diseases of unknown cause, molecules that are involved in the disease process have been identified, and can be targeted for biological therapy. Many of these molecules, which are mainly cytokines, are directly involved in the immune system. Biological therapy has found a niche in the management of cancer, autoimmune diseases, and diseases of unknown cause that result in symptoms due to immune related mechanisms.
Pegylated interferon (PEG-IFN) is a class of medication that includes three different drugs as of 2012:
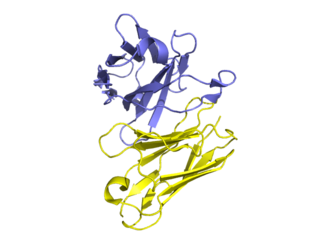
Golimumab is a human monoclonal antibody which is used as an immunosuppressive medication and sold under the brand name Simponi. Golimumab targets tumor necrosis factor alpha (TNF-alpha), a pro-inflammatory molecule and hence is a TNF inhibitor. Profound reduction in C-reactive protein (CRP) levels, interleukin (IL)-6, intercellaular adhesion molecules (ICAM)-1, matrix metalloproteinase (MMP)-3, and vascular endothelial growth factor (VEGF) demonstrates golimumab as an effective modulator of inflammatory markers and bone metabolism.
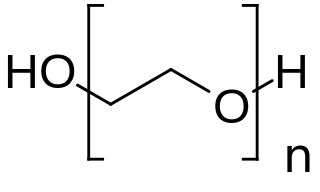
PEGylation is the process of both covalent and non-covalent attachment or amalgamation of polyethylene glycol polymer chains to molecules and macrostructures, such as a drug, therapeutic protein or vesicle, which is then described as PEGylated.

Macrogol, also known as polyethylene glycol (PEG), is used as a medication to treat constipation in children and adults. It is also used to empty the bowels before a colonoscopy. It is taken by mouth. Benefits usually occur within three days. Generally it is only recommended for up to two weeks.
Pegvisomant is a growth hormone receptor antagonist used in the treatment of acromegaly. It is primarily used if the pituitary gland tumor causing the acromegaly cannot be controlled with surgery or radiation, and the use of somatostatin analogues is unsuccessful, but is also effective as a monotherapy. It is delivered as a powder that is mixed with water and injected under the skin.

Methoxy polyethylene glycol-epoetin beta is the active ingredient of a drug marketed by Hoffmann-La Roche under the brand name Mircera. Mircera is a long-acting erythropoietin receptor activator (CERA) indicated for the treatment of patients with anaemia associated with chronic kidney disease. It is the first approved, chemically modified erythropoiesis-stimulating agent (ESA). Mircera is supplied as a solution in pre-filled syringes for intravenous or subcutaneous administration. Mircera was approved for use in Europe in July 2007 by the European Commission, in September 2007 by the Swissmedic, and in November 2007 by the U.S. Food and Drug Administration for use in the United States.
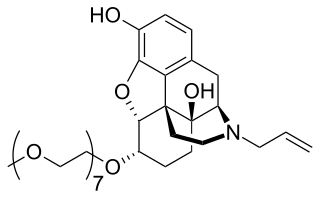
Naloxegol is a peripherally acting μ-opioid receptor antagonist developed by AstraZeneca, licensed from Nektar Therapeutics, for the treatment of opioid-induced constipation. It was approved in 2014 in adult patients with chronic, non-cancer pain. Doses of 25 mg were found safe and well tolerated for 52 weeks. When given concomitantly with opioid analgesics, naloxegol reduced constipation-related side effects, while maintaining comparable levels of analgesia.
Drug nomenclature is the systematic naming of drugs, especially pharmaceutical drugs. In the majority of circumstances, drugs have 3 types of names: chemical names, the most important of which is the IUPAC name; generic or nonproprietary names, the most important of which are the International Nonproprietary Names (INNs); and trade names, which are brand names. Under the INN system, generic names for drugs are constructed out of affixes and stems that classify the drugs into useful categories while keeping related names dinstinguishable. A marketed drug might also have a company code or compound code.
Turoctocog alfa is a recombinant antihemophilic factor VIII used for the treatment of and prophylaxis of bleeding patients with haemophilia A. It is marketed by Novo Nordisk. It was approved in the United States, the European Union, and Japan in 2013.
Nektar Therapeutics (Nektar) is an American biopharmaceutical company. The company was founded in 1990 and is based in San Francisco, California. The company develops new drug candidates by applying its proprietary PEGylation and advanced polymer conjugate technologies to modify chemical structure of substances. It is a technology supplier to a number of pharmaceutical companies including Affymax, Amgen, Merck, Pfizer and UCB Pharma, etc. The company developed the world's first inhalable non-injectable insulin, Exubera, which was awarded as the bronze award by Wall Street Journal for its technological breakthrough.
Lulizumab pegol is a monoclonal antibody designed for the treatment of autoimmune diseases.

Etirinotecan pegol is a drug developed by Nektar Therapeutics for the treatment of certain kinds of breast cancer with brain metastases. The European Medicines Agency refused to grant it a marketing authorisation in 2017.
Calaspargase pegol, sold under the brand name Asparlas, is a medication for the treatment of acute lymphoblastic leukemia (ALL). It is approved in the United States as a component of a multi-agent chemotherapeutic regimen for ALL in pediatric and young adult patients aged one month to 21 years.
Lysozyme PEGylation is the covalent attachment of Polyethylene glycol (PEG) to Lysozyme, which is one of the most widely investigated PEGylated proteins.
Damoctocog alfa pegol, sold under the brand name Jivi is a recombinant DNA-derived, Factor VIII concentrate medication used to treat hemophilia A.
References
- ↑ Swierczewska, Magdalena; Lee, Kang Choon; Lee, Seulki (2015). "What is the future of PEGylated therapies?". Expert Opinion on Emerging Drugs. 20 (4): 531–536. doi:10.1517/14728214.2015.1113254. PMC 4908577 . PMID 26583759.
| | This pharmacology-related article is a stub. You can help Wikipedia by expanding it. |