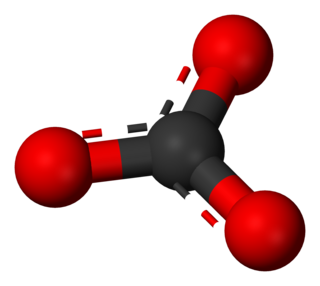
In chemistry, a carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula of CO2−
3. The name may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2.

Limestone is a carbonate sedimentary rock that is often composed of the skeletal fragments of marine organisms such as coral, foraminifera, and molluscs. Its major materials are the minerals calcite and aragonite, which are different crystal forms of calcium carbonate (CaCO3). A closely related rock is dolomite, which contains a high percentage of the mineral dolomite, CaMg(CO3)2. In fact, in old USGS publications, dolomite was referred to as magnesian limestone, a term now reserved for magnesium-deficient dolomites or magnesium-rich limestones.

Lithium carbonate is an inorganic compound, the lithium salt of carbonate with the formula Li
2CO
3. This white salt is widely used in the processing of metal oxides.

Calcium carbonate is a chemical compound with the formula CaCO3. It is a common substance found in rocks as the minerals calcite and aragonite (most notably as limestone, which is a type of sedimentary rock consisting mainly of calcite) and is the main component of pearls and the shells of marine organisms, snails, and eggs. Calcium carbonate is the active ingredient in agricultural lime and is created when calcium ions in hard water react with carbonate ions to create limescale. It is medicinally used as a calcium supplement or as an antacid, but excessive consumption can be hazardous.

The carbon cycle is the biogeochemical cycle by which carbon is exchanged among the biosphere, pedosphere, geosphere, hydrosphere, and atmosphere of the Earth. Carbon is the main component of biological compounds as well as a major component of many minerals such as limestone. Along with the nitrogen cycle and the water cycle, the carbon cycle comprises a sequence of events that are key to make Earth capable of sustaining life. It describes the movement of carbon as it is recycled and reused throughout the biosphere, as well as long-term processes of carbon sequestration to and release from carbon sinks.

Sodium carbonate, Na2CO3, (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, water-soluble salts. All forms have a strongly alkaline taste and give moderately alkaline solutions in water. Historically it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process.
Sedimentology encompasses the study of modern sediments such as sand, silt, and clay, and the processes that result in their formation, transport, deposition and diagenesis. Sedimentologists apply their understanding of modern processes to interpret geologic history through observations of sedimentary rocks and sedimentary structures.

A stalagmite is a type of rock formation that rises from the floor of a cave due to the accumulation of material deposited on the floor from ceiling drippings. Stalagmites may be composed of lava, minerals, mud, peat, pitch, sand, sinter and amberat.

Magnesium carbonate, MgCO3 (archaic name magnesia alba), is an inorganic salt that is a white solid. Several hydrated and basic forms of magnesium carbonate also exist as minerals.

Hard water is water that has high mineral content. Hard water is formed when water percolates through deposits of limestone and chalk which are largely made up of calcium and magnesium carbonates.
Calcium bicarbonate, also called calcium hydrogen carbonate, has a chemical formula Ca(HCO3)2. The term does not refer to a known solid compound; it exists only in aqueous solution containing the calcium (Ca2+), bicarbonate (HCO−
3), and carbonate (CO2−
3) ions, together with dissolved carbon dioxide (CO2). The relative concentrations of these carbon-containing species depend on the pH; bicarbonate predominates within the range 6.36–10.25 in fresh water.

Caliche is a sedimentary rock, a hardened natural cement of calcium carbonate that binds other materials—such as gravel, sand, clay, and silt. It occurs worldwide, in aridisol and mollisol soil orders—generally in arid or semiarid regions, including in central and western Australia, in the Kalahari Desert, in the High Plains of the western USA, in the Sonoran Desert and Mojave Desert, and in Eastern Saudi Arabia Al-Hasa. Caliche is also known as calcrete or kankar. It belongs to the duricrusts. The term caliche is Spanish and is originally from the Latin calx, meaning lime.

Lime is a calcium-containing inorganic mineral composed primarily of oxides, and hydroxide, usually calcium oxide and/ or calcium hydroxide. It is also the name for calcium oxide which occurs as a product of coal seam fires and in altered limestone xenoliths in volcanic ejecta. The word lime originates with its earliest use as building mortar and has the sense of sticking or adhering.

Calcareous is an adjective meaning "mostly or partly composed of calcium carbonate", in other words, containing lime or being chalky. The term is used in a wide variety of scientific disciplines.

Huygens is an impact crater on Mars named in honour of the Dutch astronomer, mathematician and physicist Christiaan Huygens.

Head (vessel) Evidence for carbonates on Mars was first discovered in 2008. Previously, most remote sensing instruments such as OMEGA and THEMIS—sensitive to infrared emissivity spectral features of carbonates—had not suggested the presence of carbonate outcrops, at least at the 100 m or coarser spatial scales available from the returned data.

Flunoxaprofen, also known as Priaxim, is a chiral nonsteroidal anti-inflammatory drug (NSAID). It is closely related to naproxen, which is also an NSAID. Flunoxaprofen has been shown to significantly improve the symptoms of osteoarthritis and rheumatoid arthritis. The clinical use of flunoxaprofen has ceased due to concerns of potential hepatotoxicity.

Lodenafil is a drug belonging to a class of drugs called PDE5 inhibitor, which many other erectile dysfunction drugs such as sildenafil, tadalafil, and vardenafil also belong to. Like udenafil and avanafil it belongs to a new generation of PDE5 inhibitors.
Richard Montanari is an American crime writer who debuted with his novel Deviant Way, published by Simon & Schuster, in 1995. It won the Online Mystery Award (OLMA) for Best First Mystery. He has since published seven more novels, which are now available in almost 30 languages.


















