Related Research Articles
The Winkler test is used to determine the concentration of dissolved oxygen in water samples. Dissolved oxygen (D.O.) is widely used in water quality studies and routine operation of water reclamation facilities to analyze its level of oxygen saturation.

An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized". Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.

Manganese dioxide is the inorganic compound with the formula MnO
2. This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for MnO
2 is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery. MnO
2 is also used as a pigment and as a precursor to other manganese compounds, such as KMnO
4. It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. MnO
2 has an α-polymorph that can incorporate a variety of atoms in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in α-MnO
2 as a possible cathode for lithium-ion batteries.

In organic chemistry, phthalic acid is an aromatic dicarboxylic acid, with formula C6H4(CO2H)2 and structure HO(O)C−C6H4−C(O)OH. Although phthalic acid is of modest commercial importance, the closely related derivative phthalic anhydride is a commodity chemical produced on a large scale. Phthalic acid is one of three isomers of benzenedicarboxylic acid, the others being isophthalic acid and terephthalic acid.

Potassium permanganate is an inorganic compound with the chemical formula KMnO4. It is a purplish-black crystalline salt, which dissolves in water as K+ and MnO−
4 ions to give an intensely pink to purple solution.
In environmental chemistry, the chemical oxygen demand (COD) is an indicative measure of the amount of oxygen that can be consumed by reactions in a measured solution. It is commonly expressed in mass of oxygen consumed over volume of solution, which in SI units is milligrams per liter (mg/L). A COD test can be used to quickly quantify the amount of organics in water. The most common application of COD is in quantifying the amount of oxidizable pollutants found in surface water or wastewater. COD is useful in terms of water quality by providing a metric to determine the effect an effluent will have on the receiving body, much like biochemical oxygen demand (BOD).

Potassium perchlorate is the inorganic salt with the chemical formula KClO4. Like other perchlorates, this salt is a strong oxidizer when the solid is heated at high temperature although it usually reacts very slowly in solution with reducing agents or organic substances. This colorless crystalline solid is a common oxidizer used in fireworks, ammunition percussion caps, explosive primers, and is used variously in propellants, flash compositions, stars, and sparklers. It has been used as a solid rocket propellant, although in that application it has mostly been replaced by the more performant ammonium perchlorate.

Potassium dichromate, K2Cr2O7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. As with all hexavalent chromium compounds, it is acutely and chronically harmful to health. It is a crystalline ionic solid with a very bright, red-orange color. The salt is popular in laboratories because it is not deliquescent, in contrast to the more industrially relevant salt sodium dichromate.
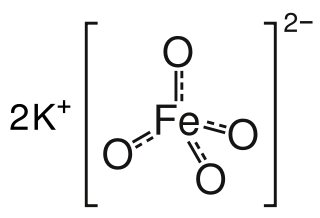
Potassium ferrate is an inorganic compound with the formula K2FeO4. It is the potassium salt of ferric acid. Potassium ferrate is a powerful oxidizing agent with applications in green chemistry, organic synthesis, and cathode technology.

A permanganate is a chemical compound with the manganate(VII) ion, MnO−
4, the conjugate base of permanganic acid. Because the manganese atom has a +7 oxidation state, the permanganate(VII) ion is a strong oxidising agent. The ion is a transition metal ion with a tetrahedral structure. Permanganate solutions are purple in colour and are stable in neutral or slightly alkaline media. The exact chemical reaction depends on the carbon-containing reactants present and the oxidant used. For example, trichloroethane (C2H3Cl3) is oxidised by permanganate ions to form carbon dioxide (CO2), manganese dioxide (MnO2), hydrogen ions (H+), and chloride ions (Cl−).

In inorganic nomenclature, a manganate is any negatively charged molecular entity with manganese as the central atom. However, the name is usually used to refer to the tetraoxidomanganate(2−) anion, MnO2−
4, also known as manganate(VI) because it contains manganese in the +6 oxidation state. Manganates are the only known manganese(VI) compounds.
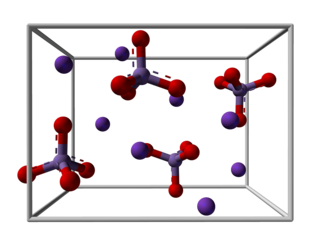
Potassium manganate is the inorganic compound with the formula K2MnO4. This green-colored salt is an intermediate in the industrial synthesis of potassium permanganate, a common chemical. Occasionally, potassium manganate and potassium permanganate are confused, but each compound's properties are distinct.
Potassium hypomanganate is the inorganic compound with the formula K3MnO4. Also known as potassium manganate(V), this bright blue solid is a rare example of a salt with the hypomanganate or manganate(V) anion, where the manganese atom is in the +5 oxidation state. It is an intermediate in the production of potassium permanganate and the industrially most important Mn(V) compound.

Ammonium permanganate is the chemical compound NH4MnO4, or NH3·HMnO4. It is a water soluble, violet-brown or dark purple salt.

Calcium permanganate is an oxidizing agent and chemical compound with the chemical formula Ca(MnO4)2. This salt consists of the metal calcium and two permanganate ions.
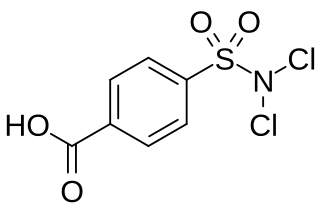
Halazone is a chemical compound whose formula can be written as either C
7H
5Cl
2NO
4S or (HOOC)(C
6H
4)(SO
2)(NCl
2). It has been widely used to disinfect drinking water.

Sodium bismuthate is an inorganic compound, and a strong oxidiser with chemical formula NaBiO3. It is somewhat hygroscopic, but not soluble in cold water, which can be convenient since the reagent can be easily removed after the reaction. It is one of the few water insoluble sodium salts. Commercial samples may be a mixture of bismuth(V) oxide, sodium carbonate and sodium peroxide.
In situ chemical oxidation (ISCO), a form of advanced oxidation process, is an environmental remediation technique used for soil and/or groundwater remediation to lower the concentrations of targeted environmental contaminants to acceptable levels. ISCO is accomplished by introducing strong chemical oxidizers into the contaminated medium to destroy chemical contaminants in place. It can be used to remediate a variety of organic compounds, including some that are resistant to natural degradation. The in situ in ISCO is just Latin for "in place", signifying that ISCO is a chemical oxidation reaction that occurs at the site of the contamination.
Barium permanganate is a chemical compound, with the formula Ba(MnO4)2. It forms violet to brown crystals that are sparingly soluble in water.
Hummers' method is a chemical process that can be used to generate graphite oxide through the addition of potassium permanganate to a solution of graphite, sodium nitrate, and sulfuric acid. It is commonly used by engineering and lab technicians as a reliable method of producing quantities of graphite oxide. It is also able to be revised in the creation of a one-atom-thick version of the substance known as graphene oxide.
References
- ↑ "Water quality -- Determination of permanganate index". ISO. Retrieved 23 November 2011.