
Whooping cough, also known as pertussis or the 100-day cough, is a highly contagious, vaccine-preventable bacterial disease. Initial symptoms are usually similar to those of the common cold with a runny nose, fever, and mild cough, but these are followed by two or three months of severe coughing fits. Following a fit of coughing, a high-pitched whoop sound or gasp may occur as the person breathes in. The violent coughing may last for 10 or more weeks, hence the phrase "100-day cough". The cough may be so hard that it causes vomiting, rib fractures, and fatigue. Children less than one year old may have little or no cough and instead have periods when they cannot breathe. The incubation period is usually seven to ten days. Disease may occur in those who have been vaccinated, but symptoms are typically milder.

An exotoxin is a toxin secreted by bacteria. An exotoxin can cause damage to the host by destroying cells or disrupting normal cellular metabolism. They are highly potent and can cause major damage to the host. Exotoxins may be secreted, or, similar to endotoxins, may be released during lysis of the cell. Gram negative pathogens may secrete outer membrane vesicles containing lipopolysaccharide endotoxin and some virulence proteins in the bounding membrane along with some other toxins as intra-vesicular contents, thus adding a previously unforeseen dimension to the well-known eukaryote process of membrane vesicle trafficking, which is quite active at the host–pathogen interface.

Bordetella bronchiseptica is a small, gram-negative, rod-shaped bacterium of the genus Bordetella. It can cause infectious bronchitis in dogs and other animals, but rarely infects humans. Closely related to B. pertussis—the obligate human pathogen that causes pertussis ; B. bronchiseptica can persist in the environment for extended periods.

The DPT vaccine or DTP vaccine is a class of combination vaccines to protect against three infectious diseases in humans: diphtheria, pertussis, and tetanus (lockjaw). The vaccine components include diphtheria and tetanus toxoids, and either killed whole cells of the bacterium that causes pertussis or pertussis antigens. The term toxoid refers to vaccines which use an inactivated toxin produced by the pathogen which they are targeted against to generate an immune response. In this way, the toxoid vaccine generates an immune response which is targeted against the toxin which is produced by the pathogen and causes disease, rather than a vaccine which is targeted against the pathogen itself. The whole cells or antigens will be depicted as either "DTwP" or "DTaP", where the lower-case "w" indicates whole-cell inactivated pertussis and the lower-case "a" stands for "acellular". In comparison to alternative vaccine types, such as live attenuated vaccines, the DTP vaccine does not contain any live pathogen, but rather uses inactivated toxoid to generate an immune response; therefore, there is not a risk of use in populations that are immune compromised since there is not any known risk of causing the disease itself. As a result, the DTP vaccine is considered a safe vaccine to use in anyone and it generates a much more targeted immune response specific for the pathogen of interest.

Bordetella is a genus of small, Gram-negative, coccobacilli bacteria of the phylum Pseudomonadota. Bordetella species, with the exception of B. petrii, are obligate aerobes, as well as highly fastidious, or difficult to culture. All species can infect humans. The first three species to be described are sometimes referred to as the 'classical species'. Two of these are also motile.

A toxoid is an inactivated toxin whose toxicity has been suppressed either by chemical (formalin) or heat treatment, while other properties, typically immunogenicity, are maintained. Toxins are secreted by bacteria, whereas toxoids are altered form of toxins; toxoids are not secreted by bacteria. Thus, when used during vaccination, an immune response is mounted and immunological memory is formed against the molecular markers of the toxoid without resulting in toxin-induced illness. Such a preparation is also known as an anatoxin. There are toxoids for prevention of diphtheria, tetanus and botulism.
Bacterial adhesins are cell-surface components or appendages of bacteria that facilitate adhesion or adherence to other cells or to surfaces, usually in the host they are infecting or living in. Adhesins are a type of virulence factor.
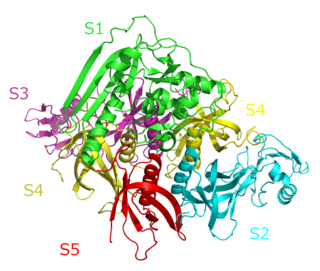
Pertussis toxin (PT) is a protein-based AB5-type exotoxin produced by the bacterium Bordetella pertussis, which causes whooping cough. PT is involved in the colonization of the respiratory tract and the establishment of infection. Research suggests PT may have a therapeutic role in treating a number of common human ailments, including hypertension, viral infection, and autoimmunity.

C5a is a protein fragment released from cleavage of complement component C5 by protease C5-convertase into C5a and C5b fragments. C5b is important in late events of the complement cascade, an orderly series of reactions which coordinates several basic defense mechanisms, including formation of the membrane attack complex (MAC), one of the most basic weapons of the innate immune system, formed as an automatic response to intrusions from foreign particles and microbial invaders. It essentially pokes microscopic pinholes in these foreign objects, causing loss of water and sometimes death. C5a, the other cleavage product of C5, acts as a highly inflammatory peptide, encouraging complement activation, formation of the MAC, attraction of innate immune cells, and histamine release involved in allergic responses. The origin of C5 is in the hepatocyte, but its synthesis can also be found in macrophages, where it may cause local increase of C5a. C5a is a chemotactic agent and an anaphylatoxin; it is essential in the innate immunity but it is also linked with the adaptive immunity. The increased production of C5a is connected with a number of inflammatory diseases.

Bordetella pertussis is a Gram-negative, aerobic, pathogenic, encapsulated coccobacillus bacterium of the genus Bordetella, and the causative agent of pertussis or whooping cough. Its virulence factors include pertussis toxin, adenylate cyclase toxin, filamentous haemagglutinin, pertactin, fimbria, and tracheal cytotoxin.
Adenylate cyclase toxin is a virulence factor produced by some members of the genus Bordetella. Together with the pertussis toxin it is the most important virulence factor of the causative agent of whooping cough, Bordetella pertussis. Bordetella bronchiseptica and Bordetella parapertussis, also able to cause pertussis-like symptoms, also produce adenylate cyclase toxin. It is a toxin secreted by the bacteria to influence the host immune system.

The Haemophilus influenzae type B vaccine, also known as Hib vaccine, is a vaccine used to prevent Haemophilus influenzae type b (Hib) infection. In countries that include it as a routine vaccine, rates of severe Hib infections have decreased more than 90%. It has therefore resulted in a decrease in the rate of meningitis, pneumonia, and epiglottitis.
M protein is a virulence factor that can be produced by certain species of Streptococcus.

Pertussis vaccine is a vaccine that protects against whooping cough (pertussis). There are two main types: whole-cell vaccines and acellular vaccines. The whole-cell vaccine is about 78% effective while the acellular vaccine is 71–85% effective. The effectiveness of the vaccines appears to decrease by between 2 and 10% per year after vaccination with a more rapid decrease with the acellular vaccines. The vaccine is only available in combination with tetanus and diphtheria vaccines. Pertussis vaccine is estimated to have saved over 500,000 lives in 2002.
The RTX toxin superfamily is a group of cytolysins and cytotoxins produced by bacteria. There are over 1000 known members with a variety of functions. The RTX family is defined by two common features: characteristic repeats in the toxin protein sequences, and extracellular secretion by the type I secretion systems (T1SS). The name RTX refers to the glycine and aspartate-rich repeats located at the C-terminus of the toxin proteins, which facilitate export by a dedicated T1SS encoded within the rtx operon.
Adenylate cyclase toxin (CyaA) is released from bacterium Bordetella pertussis by the T1SS and released in the host’s respiratory tract in order to suppress its early innate and subsequent adaptive immune defense.

In molecular biology, the haemagglutination activity domain is a conserved protein domain found near the N terminus of a number of large, repetitive bacterial proteins, including many proteins of over 2500 amino acids. A number of the members of this family have been designated adhesins, filamentous haemagglutinins, haem/haemopexin-binding protein, etc. Members generally have a signal sequence, then an intervening region, then the region described in this entry. Following this region, proteins typically have regions rich in repeats but may show no homology between the repeats of one member and the repeats of another. This domain is suggested to be a carbohydrate-dependent haemagglutination activity site.
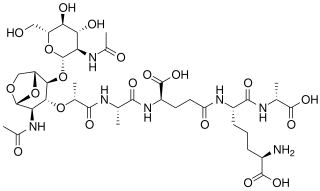
Tracheal cytotoxin (TCT) is a 921 dalton glycopeptide released by Bordetella pertussis, Vibrio fischeri, and Neisseria gonorrhoeae. It is a soluble piece of peptidoglycan (PGN) found in the cell wall of all gram-negative bacteria, but only some bacteria species release TCT due to inability to recycle this piece of anhydromuropeptide.
Vaccine resistance is the evolutionary adaptation of pathogens to infect and spread through vaccinated individuals, analogous to antimicrobial resistance. It concerns both human and animal vaccines. Although the emergence of a number of vaccine resistant pathogens has been well documented, this phenomenon is nevertheless much more rare and less of a concern than antimicrobial resistance.
Whole-cell vaccines are a type of vaccine that has been prepared in the laboratory from entire cells. Such vaccines simultaneously contain multiple antigens to activate the immune system. They induce antigen-specific T-cell responses.













