
Polyurethane refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, varnishes and coatings, adhesives, electrical potting compounds, and fibers such as spandex and PUL. Foams are the largest application accounting for 67% of all polyurethane produced in 2016.

Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low viscosity liquids. Waxes are insoluble in water but soluble in organic, nonpolar solvents. Natural waxes of different types are produced by plants and animals and occur in petroleum.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Polyethylene or polythene is the most commonly produced plastic. It is a polymer, primarily used for packaging. As of 2017, over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market.

Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.

Acrylonitrile butadiene styrene (ABS) (chemical formula (C8H8)x·(C4H6)y·(C3H3N)z) is a common thermoplastic polymer. Its glass transition temperature is approximately 105 °C (221 °F). ABS is amorphous and therefore has no true melting point.

In materials science, a thermosetting polymer, often called a thermoset, is a polymer that is obtained by irreversibly hardening ("curing") a soft solid or viscous liquid prepolymer (resin). Curing is induced by heat or suitable radiation and may be promoted by high pressure, or mixing with a catalyst. Heat is not necessarily applied externally, but is often generated by the reaction of the resin with a curing agent. Curing results in chemical reactions that create extensive cross-linking between polymer chains to produce an infusible and insoluble polymer network.
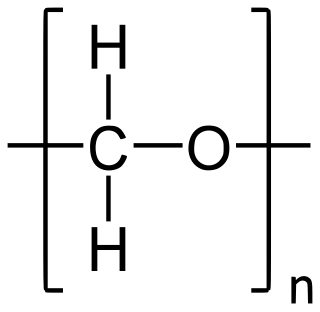
Polyoxymethylene (POM), also known as acetal, polyacetal, and polyformaldehyde, is an engineering thermoplastic used in precision parts requiring high stiffness, low friction, and excellent dimensional stability. As with many other synthetic polymers, it is produced by different chemical firms with slightly different formulas and sold variously by such names as Delrin, Kocetal, Ultraform, Celcon, Ramtal, Duracon, Kepital, Polypenco, Tenac and Hostaform.

Ethylene-vinyl acetate (EVA), also known as poly (PEVA), is the copolymer of ethylene and vinyl acetate. The weight percent of vinyl acetate usually varies from 10 to 40%, with the remainder being ethylene. There are three different types of EVA copolymer, which differ in the vinyl acetate (VA) content and the way the materials are used.

Polyphthalamide is a subset of thermoplastic synthetic resins in the polyamide (nylon) family defined as when 55% or more moles of the carboxylic acid portion of the repeating unit in the polymer chain is composed of a combination of terephthalic (TPA) and isophthalic (IPA) acids. The substitution of aliphatic diacids by aromatic diacids in the polymer backbone increases the melting point, glass transition temperature, chemical resistance and stiffness.

Hot-melt adhesive (HMA), also known as hot glue, is a form of thermoplastic adhesive that is commonly sold as solid cylindrical sticks of various diameters designed to be applied using a hot glue gun. The gun uses a continuous-duty heating element to melt the plastic glue, which the user pushes through the gun either with a mechanical trigger mechanism on the gun, or with direct finger pressure. The glue squeezed out of the heated nozzle is initially hot enough to burn and even blister skin. The glue is sticky when hot, and solidifies in a few seconds to one minute. Hot-melt adhesives can also be applied by dipping or spraying, and are popular with hobbyists and crafters both for affixing and as an inexpensive alternative to resin casting.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.
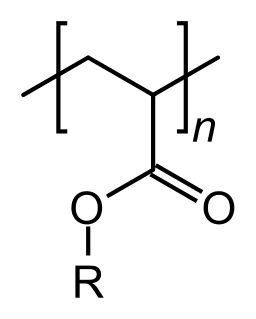
An acrylate polymer is any of a group of polymers prepared from acrylate monomers. These plastics are noted for their transparency, resistance to breakage, and elasticity.

Pressure-sensitive adhesive is a type of nonreactive adhesive which forms a bond when pressure is applied to bond the adhesive with a surface. No solvent, water, or heat is needed to activate the adhesive. It is used in pressure-sensitive tapes, labels, glue dots, stickers, sticky note pads, automobile trim, and a wide variety of other products.
Polyanhydrides are a class of biodegradable polymers characterized by anhydride bonds that connect repeat units of the polymer backbone chain. Their main application is in the medical device and pharmaceutical industry. In vivo, polyanhydrides degrade into non-toxic diacid monomers that can be metabolized and eliminated from the body. Owing to their safe degradation products, polyanhydrides are considered to be biocompatible.
Polybutylene (polybutene-1, poly(1-butene), PB-1) is a polyolefin or saturated polymer with the chemical formula (CH2CH(Et))n. Not be confused with polybutene, PB-1 is mainly used in piping.
A thermoset polymer matrix is a synthetic polymer reinforcement where polymers act as binder or matrix to secure in place incorporated particulates, fibres or other reinforcements. They were first developed for structural applications, such as glass-reinforced plastic radar domes on aircraft and graphite-epoxy payload bay doors on the Space Shuttle.
Tackifiers are chemical compounds used in formulating adhesives to increase the tack, the stickiness of the surface of the adhesive. They are usually low-molecular weight compounds with high glass transition temperature. At low strain rate, they provide higher stress compliance, and become stiffer at higher strain rates.

Polyvinylcarbazole (PVK) is a temperature-resistant thermoplastic polymer produced by radical polymerization from the monomer N-vinylcarbazole. It is a photoconductive polymer and thus the basis for photorefractive polymers and organic light-emitting diodes.














