Related Research Articles
Experimental cancer treatments are mainstream medical therapies intended to treat cancer by improving on, supplementing or replacing conventional methods. However, researchers are still trying to determine whether these treatments are safe and effective treatments. Experimental cancer treatments are normally available only to people who participate in formal research programs, which are called clinical trials. Occasionally, a seriously ill person may be able to access an experimental drug through an expanded access program. Some of the treatments have regulatory approval for treating other conditions. Health insurance and publicly funded health care programs normally refuse to pay for experimental cancer treatments.
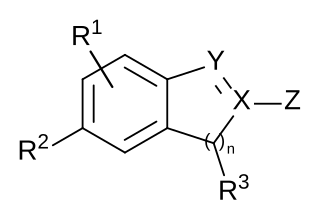
A chemical patent, pharmaceutical patent or drug patent is a patent for an invention in the chemical or pharmaceuticals industry. Strictly speaking, in most jurisdictions, there are essentially no differences between the legal requirements to obtain a patent for an invention in the chemical or pharmaceutical fields, in comparison to obtaining a patent in the other fields, such as in the mechanical field. A chemical patent or a pharmaceutical patent is therefore not a sui generis right, i.e. a special legal type of patent.

A medication is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management.

Breast milk or mother's milk is milk produced by the mammary glands in the breast of female humans. Breast milk is the primary source of nutrition for newborn infants, comprising fats, proteins, carbohydrates, and a varying composition of minerals and vitamins. Breast milk also contains substances that help protect an infant against infection and inflammation, such as symbiotic bacteria and other microorganisms and immunoglobulin A, whilst also contributing to the healthy development of the infant's immune system and gut microbiome.
Pharming, a portmanteau of farming and pharmaceutical, refers to the use of genetic engineering to insert genes that code for useful pharmaceuticals into host animals or plants that would otherwise not express those genes, thus creating a genetically modified organism (GMO). Pharming is also known as molecular farming, molecular pharming, or biopharming.

Medical research, also known as health research, refers to the process of using scientific methods with the aim to produce knowledge about human diseases, the prevention and treatment of illness, and the promotion of health.
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biologics can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine.
Test data exclusivity refers to protection of clinical trial data required to be submitted to a regulatory agency to prove safety and efficacy of a new drug, and prevention of generic drug manufacturers from relying on this data in their own applications. It provides a form of market exclusivity outside that provided by patent rights.

Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade.

Lapatinib (INN), used in the form of lapatinib ditosylate (USAN) is an orally active drug for breast cancer and other solid tumours. It is a dual tyrosine kinase inhibitor which interrupts the HER2/neu and epidermal growth factor receptor (EGFR) pathways. It is used in combination therapy for HER2-positive breast cancer. It is used for the treatment of patients with advanced or metastatic breast cancer whose tumors overexpress HER2 (ErbB2).
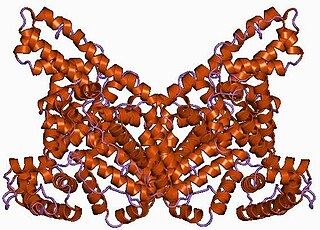
Albumin is a family of globular proteins, the most common of which are the serum albumins. All of the proteins of the albumin family are water-soluble, moderately soluble in concentrated salt solutions, and experience heat denaturation. Albumins are commonly found in blood plasma and differ from other blood proteins in that they are not glycosylated. Substances containing albumins are called albuminoids.
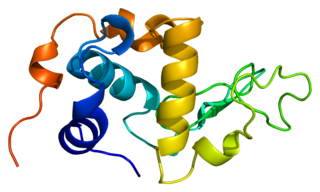
α-Lactalbumin, also known as LALBA, is a protein that in humans is encoded by the LALBA gene.

Myriad Genetics, Inc. is an American genetic testing and precision medicine company based in Salt Lake City, Utah, United States. Myriad employs a number of proprietary technologies that permit doctors and patients to understand the genetic basis of human disease and the role that genes play in the onset, progression and treatment of disease. This information is used to guide the development of new products that assess an individual's risk for developing disease later in life, identify a patient's likelihood of responding to a particular drug therapy, assess a patient's risk of disease progression and disease recurrence, and measure disease activity.

Gadobutrol (INN) (Gd-DO3A-butrol) is a gadolinium-based MRI contrast agent (GBCA).
Medication costs, also known as drug costs are a common health care cost for many people and health care systems. Prescription costs are the costs to the end consumer. Medication costs are influenced by multiple factors such as patents, stakeholder influence, and marketing expenses. A number of countries including Canada, parts of Europe, and Brazil use external reference pricing as a means to compare drug prices and to determine a base price for a particular medication. Other countries use pharmacoeconomics, which looks at the cost/benefit of a product in terms of quality of life, alternative treatments, and cost reduction or avoidance in other parts of the health care system. Structures like the UK's National Institute for Health and Clinical Excellence and to a lesser extent Canada's Common Drug Review evaluate products in this way.
The Health Impact Fund is a proposed pay-for-performance mechanism that would provide a market-based solution to problems concerning the development and distribution of medicines globally. It would incentivize the research and development of new pharmaceutical products that make substantial reductions in the global burden of disease. The Health Impact Fund is the creation of a team of researchers led by the Yale philosopher Thomas Pogge and the University of Calgary economist Aidan Hollis, and is promoted by the non-profit organization Incentives for Global Health (IGH).
HAMLET is a complex between alpha-lactalbumin and oleic acid that has been shown in cell culture experiments to induce cell death in tumor cells, but not in healthy cells.
Anticancer genes have a special ability to target and kill cancer cells without harming healthy ones. They do this through processes like programmed cell death, known as apoptosis, and other mechanisms like necrosis and autophagy. In the late 1990s, researchers discovered these genes while studying cancer cells. Sometimes, mutations or changes in these genes can occur, which might lead to cancer. These changes can include small alterations in the DNA sequence or larger rearrangements that affect the gene's function. When these anticancer genes are lost or altered, it can disrupt their ability to control cell growth, potentially leading to the development of cancer.
GcMAF is a protein produced by modification of vitamin D-binding protein. It has been falsely promoted as a treatment for various medical conditions, but claims of its benefits are not supported by evidence.
Breastmilk medicine refers to the non-nutritional usage of human breast milk (HBM) as a medicine or therapy to cure diseases. Breastmilk is perceived as an important food that provides essential nutrition to infants. It also provides protection in terms of immunity by direct transfer of antibodies from mothers to infants. The immunity developed via this mean protects infants from diseases such as respiratory diseases, middle ear infections, and gastrointestinal diseases. HBM can also produce lifelong positive therapeutic effects on a number of chronic diseases, including diabetes mellitus, obesity, hyperlipidemia, hypertension, cardiovascular diseases, autoimmunity, and asthma.
References
- ↑ Review of the Innovation Patent System (PDF). 2014. p. 2. ISBN 978-0-9804542-7-7. Archived from the original (PDF) on 2015-02-27.
- ↑ Pogge, Thomas (18 December 2011), Medicine for the 99 percent , retrieved 2015-04-15
- 1 2 Milet, Sylvain. "MedidataVoice: The Next Wave Of Pharmaceutical Innovation: An Interview With Bernard Munos". Forbes . Retrieved 2015-04-15.
- ↑ Westerling, Ragnar; Westin, Marcus; McKee, Martin; Hoffmann, Rasmus; Plug, Iris; Rey, Grégoire; Jougla, Eric; Lang, Katrin; Pärna, Kersti; Alfonso, José L.; MacKenbach, Johan P. (2014). "The timing of introduction of pharmaceutical innovations in seven European countries". Journal of Evaluation in Clinical Practice. 20 (4): 301–310. doi:10.1111/jep.12122. PMC 4282430 . PMID 24750393.
- ↑ Morgan, Steven; Lopert, Ruth; Greyson, Devon (2008). "Toward a definition of pharmaceutical innovation". Open Med. 2 (1): e4–7. PMC 3091590 . PMID 21602949.
- ↑ Feyman, Yevgeniy (13 April 2015). "Is Pharmaceutical Productivity In Decline? Maybe Not". Forbes. Retrieved 16 April 2015.
- ↑ Scannell, JW; Blanckley, A; Boldon, H; Warrington, B (2012). "Eroom's Law in pharmaceutical R&D". Nature Reviews Drug Discovery. 11 (3): 191–200. doi:10.1038/nrd3681. PMID 22378269. S2CID 3344476.
- ↑ "Review of the Innovation Patent System" (PDF). www.acip.gov.au. Archived from the original (PDF) on 2015-02-27. Retrieved 2015-04-15.
- ↑ "Archived copy" (PDF). www.unitaid.eu. Archived from the original (PDF) on 23 April 2010. Retrieved 25 January 2022.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ RUNYOWA, TAVENGWA (10 March 2011). "Medicines Patent Pool Aims To Increase Access To HIV Drugs In Developing Countries". IP Watch. Retrieved 2 June 2015.
- ↑ Hult, Kristopher. "Kristopher J. Hult: Research". University of Chicago - Department of Economics. Archived from the original on 2015-04-16. Retrieved 2015-04-16.
- ↑ "Measuring the return from pharmaceutical innovation 2012". Reuters. Archived from the original on 16 April 2015. Retrieved 16 April 2015.
- ↑ "measuring the return from pharmaceutical innovation 2014" (PDF). www2.deloitte.com. Retrieved 2015-04-15.
- ↑ Hollis, A; Grootendorst, P; Levine, DK; Pogge, T; Edwards, AM (2011). "New approaches to rewarding pharmaceutical innovation". Canadian Medical Association Journal. 183 (6): 681–5. doi:10.1503/cmaj.100375. PMC 3071389 . PMID 21149519.
- ↑ "Research and Development in the Pharmaceutical Industry". www.cbo.gov. 8 April 2021. Retrieved 2022-01-13.
- ↑ Dimasi, Joseph A; Grabowski, Henry G; Hansen, Ronald W (2016). "Innovation in the pharmaceutical industry: New estimates of R&D costs". Journal of Health Economics. 47: 20–33. doi:10.1016/j.jhealeco.2016.01.012. hdl: 10161/12742 . PMID 26928437.
- ↑ Sharples, Andrew (2011-03-23). "Gene Patents in Europe Relatively Stable Despite Uncertainty in the U.S." Genetic Engineering and Biotechnology News. Retrieved 2013-06-13.
- ↑ Sheehan, Teige. "The Supreme Court Holds Genes Are Patent-Ineligible Products of Nature" (PDF). Retrieved 20 June 2020.
- 1 2 3 Håkansson A, Zhivotovsky B, Orrenius S, Sabharwal H, Svanborg C (August 1995). "Apoptosis induced by a human milk protein". Proc. Natl. Acad. Sci. U.S.A. 92 (17): 8064–8. Bibcode:1995PNAS...92.8064H. doi: 10.1073/pnas.92.17.8064 . PMC 41287 . PMID 7644538.
- 1 2 HAMLET PHARMA. "Patents". www.hamletpharma.com. Retrieved 13 Jan 2022.
- ↑ Svensson M, Håkansson A, Mossberg AK, Linse S, Svanborg C (April 2000). "Conversion of alpha-lactalbumin to a protein inducing apoptosis". Proc. Natl. Acad. Sci. U.S.A. 97 (8): 4221–6. Bibcode:2000PNAS...97.4221S. doi: 10.1073/pnas.97.8.4221 . PMC 18203 . PMID 10760289.
- ↑ Gustafsson L, Hallgren O, Mossberg AK, Pettersson J, Fischer W, Aronsson A, Svanborg C (May 2005). "HAMLET kills tumor cells by apoptosis: structure, cellular mechanisms, and therapy". J. Nutr. 135 (5): 1299–303. doi: 10.1093/jn/135.5.1299 . PMID 15867328.
- ↑ Pettersson-Kastberg J, Aits S, Gustafsson L, Mossberg A, Storm P, Trulsson M, Persson F, Mok KH, Svanborg C (2009). "Can misfolded proteins be beneficial? The HAMLET case". Ann. Med. 41 (3): 162–76. doi:10.1080/07853890802502614. PMID 18985467. S2CID 31198109.
- ↑ HAMLET PHARMA. "About Clinical Trials". www.hamletpharma.com. Retrieved 13 Jan 2022.
- ↑ "Aloe (Aloe vera)". Mayo Clinic. 17 September 2017. Retrieved 21 January 2020.