A bactericide or bacteriocide, sometimes abbreviated Bcidal, is a substance which kills bacteria. Bactericides are disinfectants, antiseptics, or antibiotics. However, material surfaces can also have bactericidal properties based solely on their physical surface structure, as for example biomaterials like insect wings.

Dame Harriette Chick DBE was a British microbiologist, protein scientist and nutritionist. She is best remembered for demonstrating the roles of sunlight and cod liver oil in preventing rickets.

Chlorine dioxide is a chemical compound with the formula ClO2 that exists as yellowish-green gas above 11 °C, a reddish-brown liquid between 11 °C and −59 °C, and as bright orange crystals below −59 °C. It is usually handled as an aqueous solution. It is commonly used as a bleach. More recent developments have extended its applications in food processing and as a disinfectant.

A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than sterilization, which is an extreme physical or chemical process that kills all types of life. Disinfectants are generally distinguished from other antimicrobial agents such as antibiotics, which destroy microorganisms within the body, and antiseptics, which destroy microorganisms on living tissue. Disinfectants are also different from biocides—the latter are intended to destroy all forms of life, not just microorganisms. Disinfectants work by destroying the cell wall of microbes or interfering with their metabolism. It is also a form of decontamination, and can be defined as the process whereby physical or chemical methods are used to reduce the amount of pathogenic microorganisms on a surface.

Benzalkonium chloride, also known as alkyldimethylbenzylammonium chloride (ADBAC) and by the trade name Zephiran, is a type of cationic surfactant. It is an organic salt classified as a quaternary ammonium compound. ADBACs have three main categories of use: as a biocide, a cationic surfactant, and a phase transfer agent. ADBACs are a mixture of alkylbenzyldimethylammonium chlorides, in which the alkyl group has various even-numbered alkyl chain lengths.

Hypochlorous acid is a weak acid that forms when chlorine dissolves in water, and itself partially dissociates, forming hypochlorite, ClO−. HClO and ClO− are oxidizers, and the primary disinfection agents of chlorine solutions. HClO cannot be isolated from these solutions due to rapid equilibration with its precursor, chlorine.
An antimicrobial is an agent that kills microorganisms (microbicide) or stops their growth. Antimicrobial medicines can be grouped according to the microorganisms they act primarily against. For example, antibiotics are used against bacteria, and antifungals are used against fungi. They can also be classified according to their function. The use of antimicrobial medicines to treat infection is known as antimicrobial chemotherapy, while the use of antimicrobial medicines to prevent infection is known as antimicrobial prophylaxis.
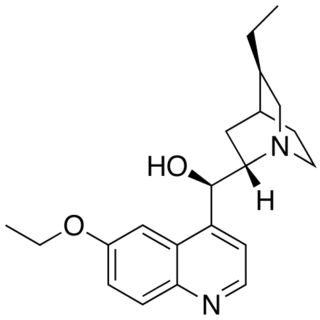
Optochin is a derivative of quinine introduced in 1911 by Morgenroth and Levy with the intention to treat pneumococci infection. In very high dilutions, it inhibits the growth of representatives of all four groups of pneumococci in vitro. That is the main reason it is now used in bacteriology for the differentiation of Streptococcus pneumoniae, which is optochin-sensitive, from the other, resistant alpha-hemolytic streptococci, sometimes called the viridans streptococci because of the green colouration on blood agar around colonies.
Triethylene glycol, TEG, or triglycol is a colorless odorless viscous liquid with molecular formula HOCH2CH2OCH2CH2OCH2CH2OH. It is used as a plasticizer for vinyl polymers. It is also used in air sanitizer products, such as "Oust" or "Clean and Pure". When aerosolized it acts as a disinfectant. Glycols are also used as liquid desiccants for natural gas and in air conditioning systems. It is an additive for hydraulic fluids and brake fluids and is used as a base for "smoke machine" fluid in the entertainment industry.
The Weil–Felix test is an agglutination test for the diagnosis of rickettsial infections. It was first described in 1916. By virtue of its long history and of its simplicity, it has been one of the most widely employed tests for rickettsia on a global scale, despite being superseded in many settings by more sensitive and specific diagnostic tests. The Weil-Felix antibody was recently found to target rickettsia LPS O-antigen.
Dakin's solution is a dilute solution of sodium hypochlorite and other stabilizing ingredients, traditionally used as an antiseptic, e.g. to cleanse wounds in order to prevent infection. The preparation was for a time called also Carrel–Dakin solution or Carrel–Dakin fluid.
The Rideal–Walker coefficient, now only of historical interest, is a figure expressing the disinfecting power of any disinfectant. It is the ratio of the dilution of the disinfectant that kills a microorganism to the dilution of phenol that kills the organism in the same time under identical conditions. The Rideal–Walker coefficient determines the phenol coefficient utilizing the method (test) described by English chemists Samuel Rideal (1863–1929) and J. T. Ainslie Walker (1868–1930).
The minimum bactericidal concentration (MBC) is the lowest concentration of an antibacterial agent required to kill a particular bacterium. It can be determined from broth dilution minimum inhibitory concentration (MIC) tests by subculturing to agar plates that do not contain the test agent. The MBC is identified by determining the lowest concentration of antibacterial agent that reduces the viability of the initial bacterial inoculum by ≥99.9%. The MBC is complementary to the MIC; whereas the MIC test demonstrates the lowest level of antimicrobial agent that inhibits growth, the MBC demonstrates the lowest level of antimicrobial agent that results in microbial death. This means that even if a particular MIC shows inhibition, plating the bacteria onto agar might still result in organism proliferation because the antimicrobial did not cause death. Antibacterial agents are usually regarded as bactericidal if the MBC is no more than four times the MIC. Because the MBC test uses colony-forming units as a proxy measure of bacterial viability, it can be confounded by antibacterial agents which cause aggregation of bacterial cells. Examples of antibacterial agents which do this include flavonoids and peptides.

Polyaminopropyl biguanide (PAPB) is a disinfectant and a preservative used for disinfection on skin and in cleaning solutions for contact lenses. It is also an ingredient in many deodorant bodysprays. It is a polymer or oligomer where biguanide functional groups are connected by propyl hydrocarbon chains. PAPB is specifically bactericidal at very low concentrations (10 mg/L) and is also fungicidal.

Bleach is the generic name for any chemical product that is used industrially or domestically to remove colour (whitening) from fabric or fiber or to clean or to remove stains in a process called bleaching. It often refers specifically to a dilute solution of sodium hypochlorite, also called "liquid bleach".
A virucide is any physical or chemical agent that deactivates or destroys viruses. The substances are not only virucidal but can be also bactericidal, fungicidal, sporicidal or tuberculocidal.
CT Values are an important part of calculating disinfectant dosage for the chlorination of drinking water. A CT value is the product of the concentration of a disinfectant and the contact time with the water being disinfected. It is typically expressed in units of mg-min/L.
Creolin is a generic name for disinfectants whose composition varies according to origin. One of its uses is as a disinfectant. It is extracted from the dry distillation of wood. The residue remaining in the autoclave vessel is a dark, syrupy mass called creosote, which is composed mainly of phenolic acid and cresylic acid. The original composition of creolin was this creosote tar oil, caustic soda, soaps, and very little water. It is of low technology and a very powerful disinfectant.
Diving equipment may be exposed to contamination in use and when this happens it must be decontaminated. This is a particular issue for hazmat diving, but incidental contamination can occur in other environments. Personal diving equipment shared by more than one user requires disinfection before use. Shared use is common for expensive commercial diving equipment, and for rental recreational equipment, and some items such as demand valves, masks, helmets and snorkels which are worn over the face or held in the mouth are possible vectors for infection by a variety of pathogens. Diving suits are also likely to be contaminated, but less likely to transmit infection directly.
Herbert Edmeston Watson was Ramsay Memorial Professor of Chemical Engineering at University College London and the inventor of the low voltage neon glow lamp.







