Aromatic compounds or arenes usually refers to organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's Rule. Aromatic compounds have the following general properties:

In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis.

In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6H5, and is often represented by the symbol Ph. The phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. A phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, the phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring.

1,4-Dichlorobenzene (1,4-DCB, p-DCB, or para-dichlorobenzene, sometimes abbreviated as PDCB or para) is an aryl chloride and isomer of dichlorobenzene with the formula C6H4Cl2. This colorless solid has a strong odor. The molecule consists of a benzene ring with two chlorine atoms (replacing hydrogen atoms) on opposing sites of the ring.

Aramid fibers, short for aromatic polyamide, are a class of heat-resistant and strong synthetic fibers. They are used in aerospace and military applications, for ballistic-rated body armor fabric and ballistic composites, in marine cordage, marine hull reinforcement, as an asbestos substitute, and in various lightweight consumer items ranging from phone cases to tennis rackets.
Twaron is a para-aramid, high-performance yarn. It is a heat-resistant fibre, helps in ballistic protection and cut protection. Twaron was developed in the early 1970s by the Dutch company Akzo Nobel's division Enka BV, later Akzo Industrial Fibers. The research name of the para-aramid fibre was originally Fiber X, but it was soon called Arenka. Although the Dutch para-aramid fiber was developed only a little later than DuPont's Kevlar, the introduction of Twaron as a commercial product came much later than Kevlar due to financial problems at the Akzo company in the 1970s. As of 2000, Twaron had become a global material and had been integrated into the global markets. Twaron has been around for over 30 years.

Poly(p-phenylene vinylene) (PPV, or polyphenylene vinylene) is a conducting polymer of the rigid-rod polymer family. PPV is the only polymer of this type that can be processed into a highly ordered crystalline thin film. PPV and its derivatives are electrically conducting upon doping. Although insoluble in water, its precursors can be manipulated in aqueous solution. The small optical band gap and its bright yellow fluorescence makes PPV a candidate in applications such as light-emitting diodes (LED) and photovoltaic devices. Moreover, PPV can be doped to form electrically conductive materials. Its physical and electronic properties can be altered by the inclusion of functional side groups.
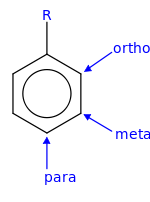
In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed.
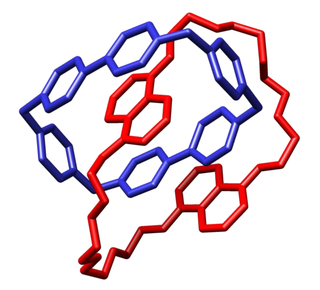
In macromolecular chemistry, a catenane is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology "mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis.
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon.

o-Phenylenediamine (OPD) is an organic compound with the formula C6H4(NH2)2. This aromatic diamine is an important precursor to many heterocyclic compounds. OPD is a white compound although samples appear darker owing to oxidation by air. It is isomeric with m-phenylenediamine and p-phenylenediamine.
Polysulfones are a family of high performance thermoplastics. These polymers are known for their toughness and stability at high temperatures. Technically used polysulfones contain an aryl-SO2-aryl subunit. Due to the high cost of raw materials and processing, polysulfones are used in specialty applications and often are a superior replacement for polycarbonates.
In organic chemistry, the Hammett equation describes a linear free-energy relationship relating reaction rates and equilibrium constants for many reactions involving benzoic acid derivatives with meta- and para-substituents to each other with just two parameters: a substituent constant and a reaction constant. This equation was developed and published by Louis Plack Hammett in 1937 as a follow-up to qualitative observations in his 1935 publication.
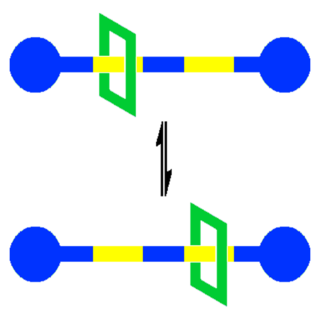
A molecular shuttle in supramolecular chemistry is a special type of molecular machine capable of shuttling molecules or ions from one location to another. This field is of relevance to nanotechnology in its quest for nanoscale electronic components and also to biology where many biochemical functions are based on molecular shuttles. Academic interest also exists for synthetic molecular shuttles, the first prototype reported in 1991 based on a rotaxane.

Phenyl ether polymers are a class of polymers that contain a phenoxy or a thiophenoxy group as the repeating group in ether linkages. Commercial phenyl ether polymers belong to two chemical classes: polyphenyl ethers (PPEs) and polyphenylene oxides (PPOs). The phenoxy groups in the former class of polymers do not contain any substituents whereas those in the latter class contain 2 to 4 alkyl groups on the phenyl ring. The structure of an oxygen-containing PPE is provided in Figure 1 and that of a 2, 6-xylenol derived PPO is shown in Figure 2. Either class can have the oxygen atoms attached at various positions around the rings.

In chemistry, inherent chirality is a property of asymmetry in molecules arising, not from a stereogenic or chiral center, but from a twisting of the molecule in 3-D space. The term was first coined by Volker Boehmer in a 1994 review, to describe the chirality of calixarenes arising from their non-planar structure in 3-D space.

Half sandwich compounds, also known as piano stool complexes, are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is a unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol.
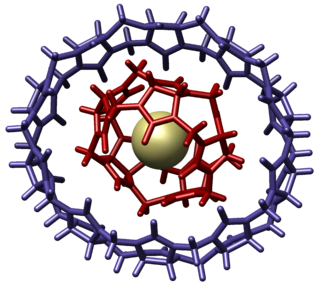
Molecular gyroscopes are chemical compounds or supramolecular complexes containing a rotor that moves freely relative to a stator, and therefore act as gyroscopes. Though any single bond or triple bond permits a chemical group to freely rotate, the compounds described as gyroscopes may protect the rotor from interactions, such as in a crystal structure with low packing density or by physically surrounding the rotor avoiding steric contact. A qualitative distinction can be made based on whether the activation energy needed to overcome rotational barriers is higher than the available thermal energy. If the activation energy required is higher than the available thermal energy, the rotor undergoes "site exchange", jumping in discrete steps between local energy minima on the potential energy surface. If there is thermal energy sufficiently higher than that needed to overcome the barrier to rotation, the molecular rotor can behave more like a macroscopic freely rotating inertial mass.
In organic chemistry, the hexadehydro-Diels–Alder (HDDA) reaction is an organic chemical reaction between a diyne and an alkyne to form a reactive benzyne species, via a [4+2] cycloaddition reaction. This benzyne intermediate then reacts with a suitable trapping agent to form a substituted aromatic product. This reaction is a derivative of the established Diels–Alder reaction and proceeds via a similar [4+2] cycloaddition mechanism. The HDDA reaction is particularly effective for forming heavily functionalized aromatic systems and multiple ring systems in one synthetic step.

Cyclobis(paraquat-p-phenylene) belongs to the class of cyclophanes, and consists of aromatic units connected by methylene bridges. It is able to incorporate small guest molecule and has played an important role in host–guest chemistry and supramolecular chemistry.