
Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested material. Endocytosis includes pinocytosis and phagocytosis. It is a form of active transport.

A lysosome is a membrane-bound organelle found in many animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules. A lysosome has a specific composition, of both its membrane proteins, and its lumenal proteins. The lumen's pH (~4.5–5.0) is optimal for the enzymes involved in hydrolysis, analogous to the activity of the stomach. Besides degradation of polymers, the lysosome is involved in various cell processes, including secretion, plasma membrane repair, apoptosis, cell signaling, and energy metabolism.
In biology, caveolae, which are a special type of lipid raft, are small invaginations of the plasma membrane in many vertebrate cell types, especially in endothelial cells, adipocytes and embryonic notochord cells. They were originally discovered by E. Yamada in 1955.

Endosomes are a collection of intracellular sorting organelles in eukaryotic cells. They are part of endocytic membrane transport pathway originating from the trans Golgi network. Molecules or ligands internalized from the plasma membrane can follow this pathway all the way to lysosomes for degradation or can be recycled back to the cell membrane in the endocytic cycle. Molecules are also transported to endosomes from the trans Golgi network and either continue to lysosomes or recycle back to the Golgi apparatus.

A liposome is a spherical vesicle having at least one lipid bilayer. The liposome can be used as a drug delivery vehicle for administration of nutrients and pharmaceutical drugs, such as lipid nanoparticles in mRNA vaccines, and DNA vaccines. Liposomes can be prepared by disrupting biological membranes.

Dendrimers are highly ordered, branched polymeric molecules. The name comes from the Greek word δένδρον (dendron) which translates to "tree". Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The word dendron is also encountered frequently. A dendron usually contains a single chemically addressable group called the focal point or core. The difference between dendrons and dendrimers is illustrated in the top figure, but the terms are typically encountered interchangeably.
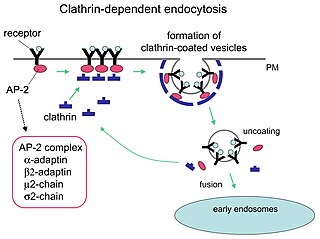
Receptor-mediated endocytosis (RME), also called clathrin-mediated endocytosis, is a process by which cells absorb metabolites, hormones, proteins – and in some cases viruses – by the inward budding of the plasma membrane (invagination). This process forms vesicles containing the absorbed substances and is strictly mediated by receptors on the surface of the cell. Only the receptor-specific substances can enter the cell through this process.
In physics and engineering, permeation is the penetration of a permeate through a solid. It is directly related to the concentration gradient of the permeate, a material's intrinsic permeability, and the materials' mass diffusivity. Permeation is modeled by equations such as Fick's laws of diffusion, and can be measured using tools such as a minipermeameter.

The osmotic-controlled release oral delivery system (OROS) is an advanced controlled release oral drug delivery system in the form of a rigid tablet with a semi-permeable outer membrane and one or more small laser drilled holes in it. As the tablet passes through the body, water is absorbed through the semipermeable membrane via osmosis, and the resulting osmotic pressure is used to push the active drug through the laser drilled opening(s) in the tablet and into the gastrointestinal tract. OROS is a trademarked name owned by ALZA Corporation, which pioneered the use of osmotic pumps for oral drug delivery.
Modified-release dosage is a mechanism that delivers a drug with a delay after its administration or for a prolonged period of time or to a specific target in the body.
Cell-penetrating peptides (CPPs) are short peptides that facilitate cellular intake and uptake of molecules ranging from nanosize particles to small chemical compounds to large fragments of DNA. The "cargo" is associated with the peptides either through chemical linkage via covalent bonds or through non-covalent interactions.

Exosomes are membrane-bound extracellular vesicles (EVs) that are produced in the endosomal compartment of most eukaryotic cells. The multivesicular body (MVB) is an endosome defined by intraluminal vesicles (ILVs) that bud inward into the endosomal lumen. If the MVB fuses with the cell surface, these ILVs are released as exosomes.
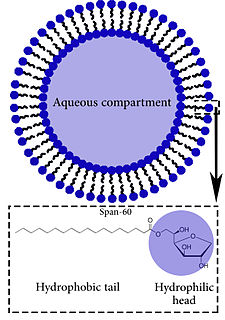
A niosome is a non-ionic surfactant-based vesicle. Niosomes are formed mostly by non-ionic surfactant and cholesterol incorporation as an excipient. Other excipients can also be used. Niosomes have more penetrating capability than the previous preparations of emulsions. They are structurally similar to liposomes in having a bilayer, however, the materials used to prepare niosomes make them more stable. It can entrap both hydrophilic and lipophilic drugs, either in an aqueous layer or in a vesicular membrane made of lipid material.
Clathrin-independent carriers (CLICs) are prevalent tubulovesicular membranes responsible for non-clathrin mediated endocytic events. They appear to endocytose material into GPI-anchored protein-enriched early endosomal compartment (GEECs). Collectively, CLICs and GEECs comprise the Cdc42-mediated CLIC/GEEC endocytic pathway, which is regulated by GRAF1.

Gene therapy utilizes the delivery of DNA into cells, which can be accomplished by several methods, summarized below. The two major classes of methods are those that use recombinant viruses and those that use naked DNA or DNA complexes.
A nanocapsule is a nanoscale shell made from a nontoxic polymer. They are vesicular systems made of a polymeric membrane which encapsulates an inner liquid core at the nanoscale. Nanocapsules have many uses, including promising medical applications for drug delivery, food enhancement, nutraceuticals, and for self-healing materials. The benefits of encapsulation methods are for protection of these substances to protect in the adverse environment, for controlled release, and for precision targeting. Nanocapsules can potentially be used as MRI-guided nanorobots or nanobots, although challenges remain.
Eps15 homology domain-containing protein 3, abbreviated as EDH3 and also known as PAST3, is a protein encoded by the EHD3 gene. It has been observed in humans, mice and rats. It belongs to the EHD protein family, a group of four membrane remodeling proteins related to the Dynamin superfamily of large GTPases. Although the four of them are 70-80% amino acid identical, they all have different locations. Its main function is related to endocytic transport.
Nanoparticle drug delivery systems are engineered technologies that use nanoparticles for the targeted delivery and controlled release of therapeutic agents. The modern form of a drug delivery system should minimize side-effects and reduce both dosage and dosage frequency. Recently, nanoparticles have aroused attention due to their potential application for effective drug delivery.

Jean Gruenberg is a Swiss biologist, and a professor at the University of Geneva. His research in the fields of cell biology and biochemistry has significantly contributed to a better understanding of the molecular mechanisms involved in the intracellular traffic within eukaryotic cells, more especially in the endolysosomal pathway.










