
Direct current (DC) is one-directional flow of electric charge. An electrochemical cell is a prime example of DC power. Direct current may flow through a conductor such as a wire, but can also flow through semiconductors, insulators, or even through a vacuum as in electron or ion beams. The electric current flows in a constant direction, distinguishing it from alternating current (AC). A term formerly used for this type of current was galvanic current.

A rectifier is an electrical device that converts alternating current (AC), which periodically reverses direction, to direct current (DC), which flows in only one direction. The reverse operation is performed by an inverter.

A flash is a device used in photography that produces a brief burst of light at a color temperature of about 5500 Kelvin to help illuminate a scene. A major purpose of a flash is to illuminate a dark scene. Other uses are capturing quickly moving objects or changing the quality of light. Flash refers either to the flash of light itself or to the electronic flash unit discharging the light. Most current flash units are electronic, having evolved from single-use flashbulbs and flammable powders. Modern cameras often activate flash units automatically.

A flashlight (US), or torch (CE) is a portable hand-held electric lamp. Formerly, the light source typically was a miniature incandescent light bulb, but these have been displaced by light-emitting diodes (LEDs) since the early 2000s. A typical flashlight consists of the light source mounted in a reflector, a transparent cover to protect the light source and reflector, a battery, and a switch, all enclosed in a case.
A photoflash capacitor is an electrolytic capacitor used in flash cameras, professional flashes, and also in solid-state laser power supplies. Their usual purpose is to briefly power a high-voltage flash tube, used to illuminate a photographic subject or optically pump a laser rod. As flash tubes require very high current for a very short time to operate, photoflash capacitors are designed to supply high discharge current pulses without excessive internal heating.
A DC-to-DC converter is an electronic circuit or electromechanical device that converts a source of direct current (DC) from one voltage level to another. It is a type of electric power converter. Power levels range from very low to very high.

An alkaline battery is a type of primary battery where the electrolyte has a pH value above 7. Typically these batteries derive energy from the reaction between zinc metal and manganese dioxide.

The AA battery is a standard size single cell cylindrical dry battery. The IEC 60086 system calls the size R6, and ANSI C18 calls it 15. It is named UM-3 by JIS of Japan. Historically, it is known as D14, U12 – later U7, or HP7 in official documentation in the United Kingdom, or a pen cell.

Zinc–air batteries (non-rechargeable), and zinc–air fuel cells are metal–air batteries powered by oxidizing zinc with oxygen from the air. These batteries have high energy densities and are relatively inexpensive to produce. Sizes range from very small button cells for hearing aids, larger batteries used in film cameras that previously used mercury batteries, to very large batteries used for electric vehicle propulsion and grid-scale energy storage.
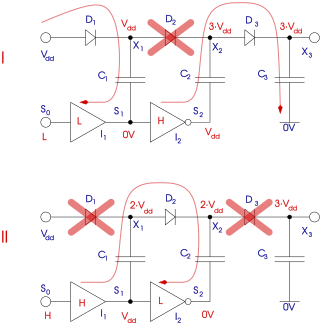
A charge pump is a kind of DC-to-DC converter that uses capacitors for energetic charge storage to raise or lower voltage. Charge-pump circuits are capable of high efficiencies, sometimes as high as 90–95%, while being electrically simple circuits.

A zinc–carbon battery (or carbon zinc battery in U.S. English) is a dry cell primary battery that provides direct electric current from the electrochemical reaction between zinc (Zn) and manganese dioxide (MnO2) in the presence of an ammonium chloride (NH4Cl) electrolyte. It produces a voltage of about 1.5 volts between the zinc anode, which is typically constructed as a cylindrical container for the battery cell, and a carbon rod surrounded by a compound with a higher Standard electrode potential (positive polarity), known as the cathode, that collects the current from the manganese dioxide electrode. The name "zinc-carbon" is slightly misleading as it implies that carbon is acting as the oxidizing agent rather than the manganese dioxide.

The nine-volt battery, or 9-volt battery, is an electric battery that supplies a nominal voltage of 9 volts. Actual voltage measures 7.2 to 9.6 volts, depending on battery chemistry. Batteries of various sizes and capacities are manufactured; a very common size is known as PP3, introduced for early transistor radios. The PP3 has a rectangular prism shape with rounded edges and two polarized snap connectors on the top. This type is commonly used for many applications including household uses such as smoke and gas detectors, clocks, and toys.

A mercury battery is a non-rechargeable electrochemical battery, a primary cell. Mercury batteries use a reaction between mercuric oxide and zinc electrodes in an alkaline electrolyte. The voltage during discharge remains practically constant at 1.35 volts, and the capacity is much greater than that of a similarly sized zinc-carbon battery. Mercury batteries were used in the shape of button cells for watches, hearing aids, cameras and calculators, and in larger forms for other applications.

A nickel–zinc battery, abbreviated NiZn, is a type of rechargeable battery similar to NiCd batteries, but with a higher voltage of 1.6 V.

The Leclanché cell is a battery invented and patented by the French scientist Georges Leclanché in 1866. The battery contained a conducting solution (electrolyte) of ammonium chloride, a cathode of carbon, a depolarizer of manganese dioxide (oxidizer), and an anode of zinc (reductant). The chemistry of this cell was later successfully adapted to manufacture a dry cell.

Batteries provided the primary source of electricity before the development of electric generators and electrical grids around the end of the 19th century. Successive improvements in battery technology facilitated major electrical advances, from early scientific studies to the rise of telegraphs and telephones, eventually leading to portable computers, mobile phones, electric cars, and many other electrical devices.

A battery–capacitor flash is a flash photography system used with flashbulbs. Instead of relying directly on the current pulse ability of a photoflash battery to directly fire a flashbulb, a battery is used to charge a capacitor that is then discharged through the flashbulb. BC flash units use 5.6 V, 15 V, or 22½ V batteries.

A lantern battery is a rectangular battery, typically an alkaline or zinc-carbon primary battery, used primarily in flashlights or lanterns. Lantern batteries are physically larger and consequently offer higher capacity than the more common flashlight batteries. Lantern batteries comprise multiple cells inside a housing.

A polymer capacitor, or more accurately a polymer electrolytic capacitor, is an electrolytic capacitor (e-cap) with a solid conductive polymer electrolyte. There are four different types:

A supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor, with a capacitance value much higher than solid-state capacitors but with lower voltage limits. It bridges the gap between electrolytic capacitors and rechargeable batteries. It typically stores 10 to 100 times more energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerates many more charge and discharge cycles than rechargeable batteries.

















