Related Research Articles

In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with a long aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, from 4 to 28. Fatty acids are a major component of the lipids in some species such as microalgae but in some other organisms are not found in their standalone form, but instead exist as three main classes of esters: triglycerides, phospholipids, and cholesteryl esters. In any of these forms, fatty acids are both important dietary sources of fuel for animals and important structural components for cells.
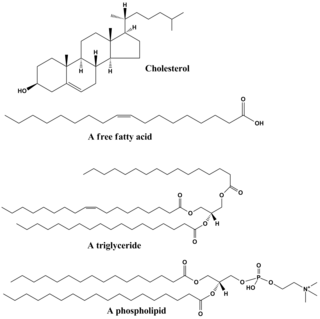
In biology and biochemistry, a lipid is a micro biomolecule that is soluble in nonpolar solvents. Non-polar solvents are typically hydrocarbons used to dissolve other naturally occurring hydrocarbon lipid molecules that do not dissolve in water, including fatty acids, waxes, sterols, fat-soluble vitamins, monoglycerides, diglycerides, triglycerides, and phospholipids.

α-Linolenic acid (ALA),, is an n−3, or omega-3, essential fatty acid. ALA is found in many seeds and oils, including flaxseed, walnuts, chia, hemp, and many common vegetable oils.

Eicosanoids are signaling molecules made by the enzymatic or non-enzymatic oxidation of arachidonic acid or other polyunsaturated fatty acids (PUFAs) that are, similar to arachidonic acid, 20 carbon units in length. Eicosanoids are a sub-category of oxylipins, i.e. oxidized fatty acids of diverse carbon units in length, and are distinguished from other oxylipins by their overwhelming importance as cell signaling molecules. Eicosanoids function in diverse physiological systems and pathological processes such as: mounting or inhibiting inflammation, allergy, fever and other immune responses; regulating the abortion of pregnancy and normal childbirth; contributing to the perception of pain; regulating cell growth; controlling blood pressure; and modulating the regional flow of blood to tissues. In performing these roles, eicosanoids most often act as autocrine signaling agents to impact their cells of origin or as paracrine signaling agents to impact cells in the proximity of their cells of origin. Eicosanoids may also act as endocrine agents to control the function of distant cells.

Lipid peroxidation is the chain of reactions of oxidative degradation of lipids. It is the process in which free radicals "steal" electrons from the lipids in cell membranes, resulting in cell damage. This process proceeds by a free radical chain reaction mechanism. It most often affects polyunsaturated fatty acids, because they contain multiple double bonds in between which lie methylene bridges (-CH2-) that possess especially reactive hydrogen atoms. As with any radical reaction, the reaction consists of three major steps: initiation, propagation, and termination. The chemical products of this oxidation are known as lipid peroxides or lipid oxidation products (LOPs).

4-Hydroxynonenal, or 4-hydroxy-2-nonenal or 4-HNE or HNE, (C9H16O2), is an α,β-unsaturated hydroxyalkenal that is produced by lipid peroxidation in cells. 4-HNE is the primary alpha,beta-unsaturated hydroxyalkenal formed in this process.
Phosphatidic acids are anionic phospholipids important to cell signaling and direct activation of lipid-gated ion channels. Hydrolysis of phosphatidic acid gives rise to one molecule each of glycerol and phosphoric acid and two molecules of fatty acids. They constitute about 0.25% of phospholipids in the bilayer.

Malondialdehyde (MDA) is the organic compound with the nominal formula CH2(CHO)2. A colorless liquid, malondialdehyde is a highly reactive compound that occurs as the enol. It occurs naturally and is a marker for oxidative stress.

Lipoxygenases are a family of (non-heme) iron-containing enzymes most of which catalyze the dioxygenation of polyunsaturated fatty acids in lipids containing a cis,cis-1,4- pentadiene into cell signaling agents that serve diverse roles as autocrine signals that regulate the function of their parent cells, paracrine signals that regulate the function of nearby cells, and endocrine signals that regulate the function of distant cells.
A fatty acid desaturase is an enzyme that removes two hydrogen atoms from a fatty acid, creating a carbon/carbon double bond. These desaturases are classified as:

Fatty aldehyde dehydrogenase is an aldehyde dehydrogenase enzyme that in human is encoded in the ALDH3A2 gene on chromosome 17. Aldehyde dehydrogenase enzymes function to remove toxic aldehydes that are generated by the metabolism of alcohol and by lipid peroxidation.

α-Parinaric acid is a conjugated polyunsaturated fatty acid. Discovered by Tsujimoto and Koyanagi in 1933, it contains 18 carbon atoms and 4 conjugated double bonds. The repeating single bond-double bond structure of α-parinaric acid distinguishes it structurally and chemically from the usual "methylene-interrupted" arrangement of polyunsaturated fatty acids that have double-bonds and single bonds separated by a methylene unit (−CH2−). Because of the fluorescent properties conferred by the alternating double bonds, α-parinaric acid is commonly used as a molecular probe in the study of biomembranes.

Nervonic acid is a fatty acid. It is a monounsaturated analog of lignoceric acid (24:0). It is also known as selacholeic acid and cis-15-tetracosenoic acid. Its name derives from the Latin word nervus, meaning nerve or sinew.
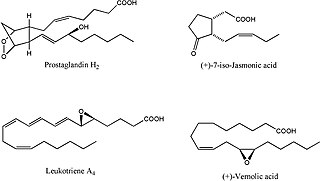
Oxylipins constitute a family of oxygenated natural products which are formed from fatty acids by pathways involving at least one step of dioxygen-dependent oxidation. Oxylipins are derived from polyunsaturated fatty acids (PUFAs) by COX enzymes (cyclooxygenases), by LOX enzymes (lipoxygenases), or by cytochrome P450 epoxygenase.
Phycotoxins are complex allelopathic chemicals produced by eukaryotic and prokaryotic algal secondary metabolic pathways. More simply, these are toxic chemicals synthesized by photosynthetic organisms. These metabolites are not harmful to the producer but may be toxic to either one or many members of the marine food web. This page focuses on phycotoxins produced by marine microalgae; however, freshwater algae and macroalgae are known phycotoxin producers and may exhibit analogous ecological dynamics. In the pelagic marine food web, phytoplankton are subjected to grazing by macro- and micro-zooplankton as well as competition for nutrients with other phytoplankton species. Marine bacteria try to obtain a share of organic carbon by maintaining symbiotic, parasitic, commensal, or predatory interactions with phytoplankton. Other bacteria will degrade dead phytoplankton or consume organic carbon released by viral lysis. The production of toxins is one strategy that phytoplankton use to deal with this broad range of predators, competitors, and parasites. Smetacek suggested that "planktonic evolution is ruled by protection and not competition. The many shapes of plankton reflect defense responses to specific attack systems". Indeed, phytoplankton retain an abundance of mechanical and chemical defense mechanisms including cell walls, spines, chain/colony formation, and toxic chemical production. These morphological and physiological features have been cited as evidence for strong predatory pressure in the marine environment. However, the importance of competition is also demonstrated by the production of phycotoxins that negatively impact other phytoplankton species. Flagellates are the principle producers of phycotoxins; however, there are known toxigenic diatoms, cyanobacteria, prymnesiophytes, and raphidophytes. Because many of these allelochemicals are large and energetically expensive to produce, they are synthesized in small quantities. However, phycotoxins are known to accumulate in other organisms and can reach high concentrations during algal blooms. Additionally, as biologically active metabolites, phycotoxins may produce ecological effects at low concentrations. These effects may be subtle, but have the potential to impact the biogeographic distributions of phytoplankton and bloom dynamics.
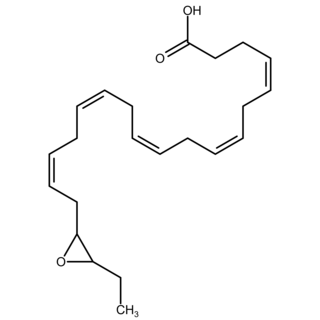
Epoxide docosapentaenoic acids are metabolites of the 22-carbon straight-chain omega-3 fatty acid, docosahexaenoic acid (DHA). Cell types that express certain cytochrome P450 (CYP) epoxygenases metabolize polyunsaturated fatty acid's (PUFAs) by converting one of their double bonds to an epoxide. In the best known of these metabolic pathways, cellular CYP epoxygenases metabolize the 20-carbon straight-chain omega-6 fatty acid, arachidonic acid, to epoxyeicosatrienoic acids (EETs); another CYP epoxygenase pathway metabolizes the 20-carbon omega-3 fatty acid, eicosapentaenoic acid (EPA), to epoxyeicosatetraenoic acids (EEQs). CYP epoxygenases similarly convert various other PUFAs to epoxides These epoxide metabolites have a variety of activities. However, essentially all of them are rapidly converted to their corresponding, but in general far less active, Vicinal (chemistry) dihydroxy fatty acids by ubiquitous cellular Soluble epoxide hydrolase. Consequently, these epoxides, including EDPs, operate as short-lived signaling agents that regulate the function of their parent or nearby cells. The particular feature of EDPs distinguishing them from EETs is that they derive from omega-3 fatty acids and are suggested to be responsible for some of the beneficial effects attributed to omega-3 fatty acids and omega-3-rich foods such as fish oil.

Epoxyeicosatetraenoic acids are a set of biologically active epoxides that various cell types make by metabolizing the omega 3 fatty acid, eicosapentaenoic acid (EPA), with certain cytochrome P450 epoxygenases. These epoxygenases can metabolize EPA to as many as 10 epoxides that differ in the site and/or stereoisomer of the epoxide formed; however, the formed EEQs, while differing in potency, often have similar bioactivities and are commonly considered together.

Hydroperoxide lyases are enzymes that catalyze the cleavage of C-C bonds in the hydroperoxides of fatty acids. They belong to the cytochrome P450 enzyme family.

Isotope effect is observed when molecules containing heavier isotopes of the same atoms are engaged in a chemical reaction at a slower rate. Deuterium-reinforced lipids can be used for the protection of living cells by slowing the chain reaction of lipid peroxidation. The lipid bilayer of the cell and organelle membranes contain polyunsaturated fatty acids (PUFA) are key components of cell and organelle membranes. Any process that either increases oxidation of PUFAs or hinders their ability to be replaced can lead to serious disease. Correspondingly, drugs that stop the chain reaction of lipid peroxidation have preventive and therapeutic potential.

Reinforced lipids are lipid molecules in which some of the fatty acids contain deuterium instead of hydrogen. They can be used for the protection of living cells by slowing the chain reaction due to isotope effect on lipid peroxidation. The lipid bilayer of the cell and organelle membranes contain polyunsaturated fatty acids (PUFA) are key components of cell and organelle membranes. Any process that either increases oxidation of PUFAs or hinders their ability to be replaced can lead to serious disease. Correspondingly, use of reinforced lipids that stop the chain reaction of lipid peroxidation has preventive and therapeutic potential.
References
- 1 2 Miller, Charles B. Biological Oceanography. Malden, MA: Blackwell Pub., 2004. Print.
- ↑ Solomons, T. W. Graham., and Craig B. Fryhle (2008). Organic Chemistry. Hoboken, NJ: John Wiley.CS1 maint: multiple names: authors list (link)
- ↑ Schilmiller, Anthony L.; Howe, Gregg A. (2005). "Systemic signaling in the wound response". Current Opinion in Plant Biology. 8 (4): 369–377. doi:10.1016/j.pbi.2005.05.008. PMID 15939667.
- ↑ Fontana, Angelo; d'Ippolito, Giuliana; Cutignano, Adele; Miralto, Antonio; Ianora, Adrianna; Romano, Giovanna; Cimino, Guido (2007). "Chemistry of oxylipin pathways in marine diatoms". Pure and Applied Chemistry. 79 (4): 481–490. doi:10.1351/pac200779040481. S2CID 53393465.
- ↑ Ribalet, François; Bastianini, Mauro; Vidoudez, Charles; Acri, Francesco; Berges, John; Ianora, Adrianna; Miralto, Antonio; Pohnert, Georg; Romano, Giovanna; Wichard, Thomas; Casotti, Raffaella (2014). "Phytoplankton Cell Lysis Associated with Polyunsaturated Aldehyde Release in the Northern Adriatic Sea". PLOS ONE. 9 (1): e85947. Bibcode:2014PLoSO...985947R. doi: 10.1371/journal.pone.0085947 . PMC 3908894 . PMID 24497933.
- ↑ Barofsky, Alexandra; Pohnert, Georg (2007). "Biosynthesis of Polyunsaturated Short Chain Aldehydes in the Diatom Thalassiosira rotula". Organic Letters. 9 (6): 1017–1020. doi:10.1021/ol063051v. PMID 17298073.
- 1 2 Wichard, Thomas; Gerecht, Andrea; Boersma, Maarten; Poulet, Serge A.; Wiltshire, Karen; Pohnert, Georg (2007). "Lipid and Fatty Acid Composition of Diatoms Revisited: Rapid Wound-Activated Change of Food Quality Parameters Influences Herbivorous Copepod Reproductive Success" (PDF). ChemBioChem. 8 (10): 1146–1153. doi:10.1002/cbic.200700053. PMID 17541989. S2CID 1085694.
- 1 2 3 Lavrentyev, Peter; Franzè, Gayantonia; Pierson, James; Stoecker, Diane (2015). "The Effect of Dissolved Polyunsaturated Aldehydes on Microzooplankton Growth Rates in the Chesapeake Bay and Atlantic Coastal Waters". Marine Drugs. 13 (5): 2834–2856. doi: 10.3390/md13052834 . PMC 4446608 . PMID 25955757.
- ↑ Miralto, A.; Barone, G.; Romano, G.; Poulet, S. A.; Ianora, A.; Russo, G. L.; Buttino, I.; Mazzarella, G.; Laabir, M.; Cabrini, M.; Giacobbe, M. G. (1999). "The insidious effect of diatoms on copepod reproduction". Nature. 402 (6758): 173–176. Bibcode:1999Natur.402..173M. doi:10.1038/46023. S2CID 4318896.