Related Research Articles
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally for the purpose of removing impurities or volatile substances and/or to incur thermal decomposition.

Piperazine is an organic compound that consists of a six-membered ring containing two nitrogen atoms at opposite positions in the ring. Piperazine exists as small alkaline deliquescent crystals with a saline taste.
Pyrometallurgy is a branch of extractive metallurgy. It consists of the thermal treatment of minerals and metallurgical ores and concentrates to bring about physical and chemical transformations in the materials to enable recovery of valuable metals. Pyrometallurgical treatment may produce products able to be sold such as pure metals, or intermediate compounds or alloys, suitable as feed for further processing. Examples of elements extracted by pyrometallurgical processes include the oxides of less reactive elements like iron, copper, zinc, chromium, tin, and manganese.
Amine gas treating, also known as amine scrubbing, gas sweetening and acid gas removal, refers to a group of processes that use aqueous solutions of various alkylamines (commonly referred to simply as amines) to remove hydrogen sulfide (H2S) and carbon dioxide (CO2) from gases. It is a common unit process used in refineries, and is also used in petrochemical plants, natural gas processing plants and other industries.

Flue gas is the gas exiting to the atmosphere via a flue, which is a pipe or channel for conveying exhaust gases, as from a fireplace, oven, furnace, boiler or steam generator. It often refers to the exhaust gas of combustion at power plants. Technology is available to remove pollutants from flue gas at power plants.

Coal pollution mitigation, sometimes labeled as clean coal, is a series of systems and technologies that seek to mitigate health and environmental impact of burning coal for energy. Burning coal releases harmful substances that contribute to air pollution, acid rain, and greenhouse gas emissions. Mitigation includes precombustion approaches, such as cleaning coal, and post combustion approaches, include flue-gas desulfurization, selective catalytic reduction, electrostatic precipitators, and fly ash reduction. These measures aim to reduce coal's impact on human health and the environment.

Carbon capture and storage (CCS) is a process in which a relatively pure stream of carbon dioxide (CO2) from industrial sources is separated, treated and transported to a long-term storage location. For example, the burning of fossil fuels or biomass results in a stream of CO2 that could be captured and stored by CCS. Usually the CO2 is captured from large point sources, such as a chemical plant or a bioenergy plant, and then stored in a suitable geological formation. The aim is to reduce greenhouse gas emissions and thus mitigate climate change. For example, CCS retrofits for existing power plants can be one of the ways to limit emissions from the electricity sector and meet the Paris Agreement goals.
An integrated gasification combined cycle (IGCC) is a technology using a high pressure gasifier to turn coal and other carbon based fuels into pressurized gas—synthesis gas (syngas). It can then remove impurities from the syngas prior to the electricity generation cycle. Some of these pollutants, such as sulfur, can be turned into re-usable byproducts through the Claus process. This results in lower emissions of sulfur dioxide, particulates, mercury, and in some cases carbon dioxide. With additional process equipment, a water-gas shift reaction can increase gasification efficiency and reduce carbon monoxide emissions by converting it to carbon dioxide. The resulting carbon dioxide from the shift reaction can be separated, compressed, and stored through sequestration. Excess heat from the primary combustion and syngas fired generation is then passed to a steam cycle, similar to a combined cycle gas turbine. This process results in improved thermodynamic efficiency, compared to conventional pulverized coal combustion.
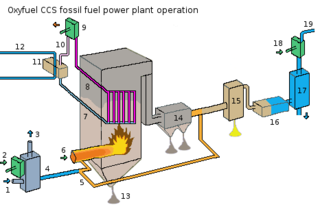
Oxy-fuel combustion is the process of burning a fuel using pure oxygen, or a mixture of oxygen and recirculated flue gas, instead of air. Since the nitrogen component of air is not heated, fuel consumption is reduced, and higher flame temperatures are possible. Historically, the primary use of oxy-fuel combustion has been in welding and cutting of metals, especially steel, since oxy-fuel allows for higher flame temperatures than can be achieved with an air-fuel flame. It has also received a lot of attention in recent decades as a potential carbon capture and storage technology.

A carbon dioxide scrubber is a piece of equipment that absorbs carbon dioxide (CO2). It is used to treat exhaust gases from industrial plants or from exhaled air in life support systems such as rebreathers or in spacecraft, submersible craft or airtight chambers. Carbon dioxide scrubbers are also used in controlled atmosphere (CA) storage. They have also been researched for carbon capture and storage as a means of combating climate change.

Chemical looping combustion (CLC) is a technological process typically employing a dual fluidized bed system. CLC operated with an interconnected moving bed with a fluidized bed system, has also been employed as a technology process. In CLC, a metal oxide is employed as a bed material providing the oxygen for combustion in the fuel reactor. The reduced metal is then transferred to the second bed and re-oxidized before being reintroduced back to the fuel reactor completing the loop. Fig 1 shows a simplified diagram of the CLC process. Fig 2 shows an example of a dual fluidized bed circulating reactor system and a moving bed-fluidized bed circulating reactor system.

Methyldiethanolamine, also known as N-methyl diethanolamine and more commonly as MDEA, is the organic compound with the formula CH3N(C2H4OH)2. It is a colorless liquid with an ammonia odor. It is miscible with water, ethanol and benzene. A tertiary amine, it is widely used as a sweetening agent in chemical, oil refinery, syngas production and natural gas.
Carbon capture and storage (CCS) is a technology that can capture carbon dioxide CO2 emissions produced from fossil fuels in electricity, industrial processes which prevents CO2 from entering the atmosphere. Carbon capture and storage is also used to sequester CO2 filtered out of natural gas from certain natural gas fields. While typically the CO2 has no value after being stored, Enhanced Oil Recovery uses CO2 to increase yield from declining oil fields.

Bioenergy with carbon capture and storage (BECCS) is the process of extracting bioenergy from biomass and capturing and storing the carbon, thereby removing it from the atmosphere. BECCS can theoretically be a "negative emissions technology" (NET), although its deployment at the scale considered by many governments and industries can "also pose major economic, technological, and social feasibility challenges; threaten food security and human rights; and risk overstepping multiple planetary boundaries, with potentially irreversible consequences". The carbon in the biomass comes from the greenhouse gas carbon dioxide (CO2) which is extracted from the atmosphere by the biomass when it grows. Energy ("bioenergy") is extracted in useful forms (electricity, heat, biofuels, etc.) as the biomass is utilized through combustion, fermentation, pyrolysis or other conversion methods.
Calcium looping (CaL), or the regenerative calcium cycle (RCC), is a second-generation carbon capture technology. It is the most developed form of carbonate looping, where a metal (M) is reversibly reacted between its carbonate form (MCO3) and its oxide form (MO) to separate carbon dioxide from other gases coming from either power generation or an industrial plant. In the calcium looping process, the two species are calcium carbonate (CaCO3) and calcium oxide (CaO). The captured carbon dioxide can then be transported to a storage site, used in enhanced oil recovery or used as a chemical feedstock. Calcium oxide is often referred to as the sorbent.
The use of ionic liquids in carbon capture is a potential application of ionic liquids as absorbents for use in carbon capture and sequestration. Ionic liquids, which are salts that exist as liquids near room temperature, are polar, nonvolatile materials that have been considered for many applications. The urgency of climate change has spurred research into their use in energy-related applications such as carbon capture and storage.

Carbon capture and utilization (CCU) is the process of capturing carbon dioxide (CO2) from industrial processes and transporting it via pipelines to where one intends to use it in industrial processes.

Direct air capture (DAC) is the use of chemical or physical processes to extract carbon dioxide directly from the ambient air. If the extracted CO2 is then sequestered in safe long-term storage, the overall process will achieve carbon dioxide removal and be a "negative emissions technology" (NET).
Hot potassium carbonate, HPC, is a method used to remove carbon dioxide from gas mixtures, in some contexts referred to as carbon scrubbing. The inorganic, basic compound potassium carbonate is mixed with a gas mixture and the liquid absorbs carbon dioxide through chemical processes. The technology is a form of chemical absorption, and was developed for natural gas sweetening (i.e., removal of acidic from raw natural gas). Currently it is also considered, among others, as a post-combustion capture process, in the contexts of carbon capture and storage and carbon capture and utilization. As a post-combustion CO2 capture process, the technology is planned to be used on full scale on a heat plant in Stockholm from 2025.
References
- 1 2 "Post-combustion carbon dioxide capture: best available techniques (BAT)" . Retrieved 15 November 2023.
- ↑ "Home". Advanced Indirectly Heated Carbonate Looping Process. Retrieved 23 June 2020.