Petrochemicals (sometimes abbreviated as petchems [1] ) are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.
Contents
The two most common petrochemical classes are olefins (including ethylene and propylene) and aromatics (including benzene, toluene and xylene isomers).
Oil refineries produce olefins and aromatics by fluid catalytic cracking of petroleum fractions. Chemical plants produce olefins by steam cracking of natural gas liquids like ethane and propane. Aromatics are produced by catalytic reforming of naphtha. Olefins and aromatics are the building-blocks for a wide range of materials such as solvents, detergents, and adhesives. Olefins are the basis for polymers and oligomers used in plastics, resins, fibers, elastomers, lubricants, and gels. [2] [3]
Global ethylene production was 190 million tonnes and propylene was 120 million tonnes in 2019. [4] Aromatics production is approximately 70 million tonnes. The largest petrochemical industries are located in the United States and Western Europe; however, major growth in new production capacity is in the Middle East and Asia. There is substantial inter-regional petrochemical trade.
Primary petrochemicals are divided into three groups depending on their chemical structure:
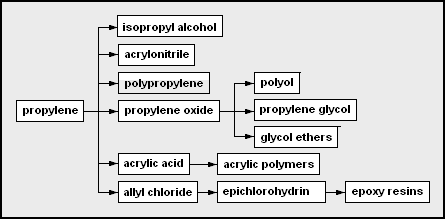
- Olefins includes ethene, propene, butenes and butadiene. Ethylene and propylene are important sources of industrial chemicals and plastics products. Butadiene is used in making synthetic rubber.
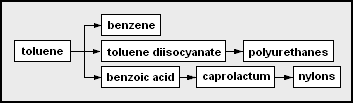
- Aromatics includes benzene, toluene and xylenes, as a whole referred to as BTX and primarily obtained from petroleum refineries by extraction from the reformate produced in catalytic reformers using naphtha obtained from petroleum refineries. Alternatively, BTX can be produced by aromatization of alkanes. Benzene is a raw material for dyes and synthetic detergents, and benzene and toluene for isocyanates MDI and TDI used in making polyurethanes. Manufacturers use xylenes to produce plastics and synthetic fibers.
- Synthesis gas is a mixture of carbon monoxide and hydrogen used to produce methanol and other chemicals. Steam crackers are not to be confused with steam reforming plants used to produce hydrogen for ammonia production. Ammonia is used to make the fertilizer urea and methanol is used as a solvent and chemical intermediate.
- Methane, ethane, propane and butanes obtained primarily from natural gas processing plants.
- Methanol and formaldehyde.
In 2007, the amounts of ethylene and propylene produced in steam crackers were about 115 Mt (megatonnes) and 70 Mt, respectively. [5] The output ethylene capacity of large steam crackers ranged up to as much as 1.0 – 1.5 Mt per year. [6]
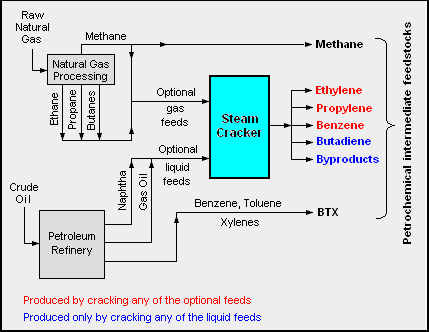
The adjacent diagram schematically depicts the major hydrocarbon sources and processes used in producing petrochemicals. [2] [3] [7] [8]
Like commodity chemicals, petrochemicals are made on a very large scale. Petrochemical manufacturing units differ from commodity chemical plants in that they often produce a number of related products. Compare this with specialty chemical and fine chemical manufacture where products are made in discrete batch processes.
Petrochemicals are predominantly made in a few manufacturing locations around the world, for example in Jubail and Yanbu Industrial Cities in Saudi Arabia, Texas and Louisiana in the US, in Teesside in the Northeast of England in the United Kingdom, in Tarragona in Catalonia, in Rotterdam in the Netherlands, in Antwerp in Belgium, in Jamnagar, Dahej in Gujarat, India and in Singapore. Not all of the petrochemical or commodity chemical materials produced by the chemical industry are made in one single location but groups of related materials are often made in adjacent manufacturing plants to induce industrial symbiosis as well as material and utility efficiency and other economies of scale. This is known in chemical engineering terminology as integrated manufacturing. Specialty and fine chemical companies are sometimes found in similar manufacturing locations as petrochemicals but, in most cases, they do not need the same level of large-scale infrastructure (e.g., pipelines, storage, ports, and power, etc.) and therefore can be found in multi-sector business parks.
The large-scale petrochemical manufacturing locations have clusters of manufacturing units that share utilities and large-scale infrastructures such as power stations, storage tanks, port facilities, road and rail terminals. In the United Kingdom, for example, there are four main locations for such manufacturing: near the River Mersey in North West England, on the Humber on the East coast of Yorkshire, in Grangemouth near the Firth of Forth in Scotland, and in Teesside as part of the Northeast of England Process Industry Cluster (NEPIC). To demonstrate the clustering and integration, some 50% of the United Kingdom's petrochemical and commodity chemicals are produced by the NEPIC industry cluster companies in Teesside.