Related Research Articles

Electrochemistry is the branch of physical chemistry concerned with the relationship between electrical potential difference and identifiable chemical change. These reactions involve electrons moving via an electronically-conducting phase between electrodes separated by an ionically conducting and electronically insulating electrolyte.

An electrochemical cell is a device that generates electrical energy from chemical reactions. Electrical energy can also be applied to these cells to cause chemical reactions to occur. Electrochemical cells that generate an electric current are called voltaic or galvanic cells and those that generate chemical reactions, via electrolysis for example, are called electrolytic cells.

In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.

A pH meter is a scientific instrument that measures the hydrogen-ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH. The pH meter measures the difference in electrical potential between a pH electrode and a reference electrode, and so the pH meter is sometimes referred to as a "potentiometric pH meter". The difference in electrical potential relates to the acidity or pH of the solution. Testing of pH via pH meters (pH-metry) is used in many applications ranging from laboratory experimentation to quality control.
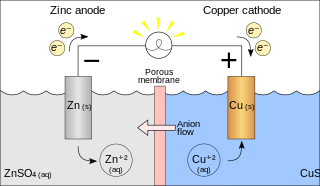
A galvanic cell or voltaic cell, named after the scientists Luigi Galvani and Alessandro Volta, respectively, is an electrochemical cell in which an electric current is generated from spontaneous oxidation–reduction reactions. A common apparatus generally consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a porous membrane.

In electrochemistry, cyclic voltammetry (CV) is a type of potentiodynamic measurement. In a cyclic voltammetry experiment, the working electrode potential is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles of ramps in potential may be repeated as many times as needed. The current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode.
A silver chloride electrode is a type of reference electrode, commonly used in electrochemical measurements. For environmental reasons it has widely replaced the saturated calomel electrode. For example, it is usually the internal reference electrode in pH meters and it is often used as reference in reduction potential measurements. As an example of the latter, the silver chloride electrode is the most commonly used reference electrode for testing cathodic protection corrosion control systems in sea water environments.

An ion-sensitive field-effect transistor (ISFET) is a field-effect transistor used for measuring ion concentrations in solution; when the ion concentration (such as H+, see pH scale) changes, the current through the transistor will change accordingly. Here, the solution is used as the gate electrode. A voltage between substrate and oxide surfaces arises due to an ion sheath. It is a special type of MOSFET (metal–oxide–semiconductor field-effect transistor), and shares the same basic structure, but with the metal gate replaced by an ion-sensitive membrane, electrolyte solution and reference electrode. Invented in 1970, the ISFET was the first biosensor FET (BioFET).

Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical data for a voltammetric experiment comes in the form of a voltammogram, which plots the current produced by the analyte versus the potential of the working electrode.
A ChemFET is a chemically-sensitive field-effect transistor, that is a field-effect transistor used as a sensor for measuring chemical concentrations in solution. When the target analyte concentration changes, the current through the transistor will change accordingly. Here, the analyte solution separates the source and gate electrodes. A concentration gradient between the solution and the gate electrode arises due to a semi-permeable membrane on the FET surface containing receptor moieties that preferentially bind the target analyte. This concentration gradient of charged analyte ions creates a chemical potential between the source and gate, which is in turn measured by the FET.
In battery technology, a concentration cell is a limited form of a galvanic cell that has two equivalent half-cells of the same composition differing only in concentrations. One can calculate the potential developed by such a cell using the Nernst equation. A concentration cell produces a small voltage as it attempts to reach chemical equilibrium, which occurs when the concentration of reactant in both half-cells are equal. Because an order of magnitude concentration difference produces less than 60 millivolts at room temperature, concentration cells are not typically used for energy storage.
In analytical chemistry, potentiometric titration is a technique similar to direct titration of a redox reaction. It is a useful means of characterizing an acid. No indicator is used; instead the electric potential is measured across the analyte, typically an electrolyte solution. To do this, two electrodes are used, an indicator electrode and a reference electrode. Reference electrodes generally used are hydrogen electrodes, calomel electrodes, and silver chloride electrodes. The indicator electrode forms an electrochemical half-cell with the interested ions in the test solution. The reference electrode forms the other half-cell.
Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. These methods can be broken down into several categories depending on which aspects of the cell are controlled and which are measured. The four main categories are potentiometry, amperometry, coulometry, and voltammetry.
A fluoride selective electrode is a type of ion selective electrode sensitive to the concentration of the fluoride ion. A common example is the lanthanum fluoride electrode.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
Bulk electrolysis is also known as potentiostatic coulometry or controlled potential coulometry. The experiment is a form of coulometry which generally employs a three electrode system controlled by a potentiostat. In the experiment the working electrode is held at a constant potential (volts) and current (amps) is monitored over time (seconds). In a properly run experiment an analyte is quantitatively converted from its original oxidation state to a new oxidation state, either reduced or oxidized. As the substrate is consumed, the current also decreases, approaching zero when the conversion nears completion.

Conductivity of an electrolyte solution is a measure of its ability to conduct electricity. The SI unit of conductivity is siemens per meter (S/m).
A biotransducer is the recognition-transduction component of a biosensor system. It consists of two intimately coupled parts; a bio-recognition layer and a physicochemical transducer, which acting together converts a biochemical signal to an electronic or optical signal. The bio-recognition layer typically contains an enzyme or another binding protein such as antibody. However, oligonucleotide sequences, sub-cellular fragments such as organelles and receptor carrying fragments, single whole cells, small numbers of cells on synthetic scaffolds, or thin slices of animal or plant tissues, may also comprise the bio-recognition layer. It gives the biosensor selectivity and specificity. The physicochemical transducer is typically in intimate and controlled contact with the recognition layer. As a result of the presence and biochemical action of the analyte, a physico-chemical change is produced within the biorecognition layer that is measured by the physicochemical transducer producing a signal that is proportionate to the concentration of the analyte. The physicochemical transducer may be electrochemical, optical, electronic, gravimetric, pyroelectric or piezoelectric. Based on the type of biotransducer, biosensors can be classified as shown to the right.

Electrochemical stripping analysis is a set of analytical chemistry methods based on voltammetry or potentiometry that are used for quantitative determination of ions in solution. Stripping voltammetry have been employed for analysis of organic molecules as well as metal ions. Carbon paste, glassy carbon paste, and glassy carbon electrodes when modified are termed as chemically modified electrodes and have been employed for the analysis of organic and inorganic compounds.
References
- ↑ "Oxygen sensor". freepatentsonline.com.