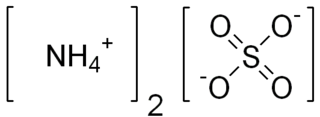A Southern blot is a method used in molecular biology for detection of a specific DNA sequence in DNA samples. Southern blotting combines transfer of electrophoresis-separated DNA fragments to a filter membrane and subsequent fragment detection by probe hybridization.
The word at is an English word, which may act as a preposition.
CAD is a commonly used acronym for computer-aided design.

Orography is the study of the topographic relief of mountains, and can more broadly include hills, and any part of a region's elevated terrain. Orography falls within the broader discipline of geomorphology.

In meteorology, a virga is an observable streak or shaft of precipitation falling from a cloud that evaporates or sublimates before reaching the ground. A shaft of precipitation that does not evaporate before reaching the ground is a precipitation shaft. At high altitudes the precipitation falls mainly as ice crystals before melting and finally evaporating; this is often due to compressional heating, because the air pressure increases closer to the ground. It is very common in deserts and temperate climates. In North America, it is commonly seen in the Western United States and the Canadian Prairies. It is also very common in the Middle East, Australia, and North Africa.
Diuresis is increased urination (polyuria) or, in the related word senses more often intended, the physiologic process that produces such an increase or the administration of medications to encourage that process. It involves extra urine production in the kidneys as part of the body's homeostatic maintenance of fluid balance.

In biochemistry, immunostaining is any use of an antibody-based method to detect a specific protein in a sample. The term "immunostaining" was originally used to refer to the immunohistochemical staining of tissue sections, as first described by Albert Coons in 1941. However, immunostaining now encompasses a broad range of techniques used in histology, cell biology, and molecular biology that use antibody-based staining methods.

Alkaline phosphatase, or basic phosphatase, is a homodimeric protein enzyme of 86 kilodaltons. Each monomer contains five cysteine residues, two zinc atoms and one magnesium atom crucial to its catalytic function, and it is optimally active at alkaline pH environments.
Salting out is a purification technique that utilizes the reduced solubility of certain molecules in a solution of very high ionic strength. Salting out is typically used to precipitate large biomolecules, such as proteins or DNA. Because the salt concentration needed for a given protein to precipitate out of the solution differs from protein to protein, a specific salt concentration can be used to precipitate a target protein. This process is also used to concentrate dilute solutions of proteins. Dialysis can be used to remove the salt if needed.
The first isolation of deoxyribonucleic acid (DNA) was done in 1869 by Friedrich Miescher. Currently it is a routine procedure in molecular biology or forensic analyses. For the chemical method, there are many different kits used for extraction, and selecting the correct one will save time on kit optimization and extraction procedures. PCR sensitivity detection is considered to show the variation between the commercial kits.
In physics, the phase problem is the problem of loss of information concerning the phase that can occur when making a physical measurement. The name comes from the field of X-ray crystallography, where the phase problem has to be solved for the determination of a structure from diffraction data. The phase problem is also met in the fields of imaging and signal processing. Various approaches of phase retrieval have been developed over the years.
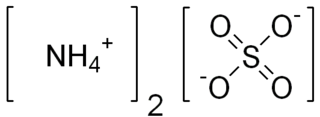
Ammonium sulfate (American English and international scientific usage; ammonium sulphate in British English); (NH4)2SO4, is an inorganic salt with a number of commercial uses. The most common use is as a soil fertilizer. It contains 21% nitrogen and 24% sulfur.

A plasmid preparation is a method of DNA extraction and purification for plasmid DNA. Many methods have been developed to purify plasmid DNA from bacteria. These methods invariably involve three steps:
IPO usually refers to an initial public offering.

Alkaline precipitation occurs due to natural and anthropogenic causes. It happens when minerals, such as calcium, aluminum, or magnesium combine with other minerals to form alkaline residues that are emitted into the atmosphere, absorbed by water droplets in clouds, and eventually fall as rain. Aquatic environments are especially impacted by alkaline precipitation. Because alkaline precipitation can be harmful to the environment, it is important to utilize various methods such as air pollution control, solidification and stabilization, and remediation to manage it.

Toluidine blue, also known as TBO or tolonium chloride (INN) is a blue cationic (basic) dye used in histology and sometimes clinically.
Electron precipitation is an atmospheric phenomenon that occurs when previously trapped electrons enter the Earth's atmosphere, thus creating communications interferences and other disturbances. Electrons trapped by Earth's magnetic field spiral around field lines to form the Van Allen radiation belt. The electrons are from the solar wind and may remain trapped above Earth for an indefinite period of time. When broadband very low frequency (VLF) waves propagate the radiation belts, the electrons exit the radiation belt and "precipitate" into the ionosphere where the electrons will collide with ions. Electron precipitation is regularly linked to ozone depletion. It is often caused by lightning strikes.
This page is based on this
Wikipedia article Text is available under the
CC BY-SA 4.0 license; additional terms may apply.
Images, videos and audio are available under their respective licenses.