
A carcinogen is any substance, radionuclide, or radiation that promotes carcinogenesis, the formation of cancer. This may be due to the ability to damage the genome or to the disruption of cellular metabolic processes. Several radioactive substances are considered carcinogens, but their carcinogenic activity is attributed to the radiation, for example gamma rays and alpha particles, which they emit. Common examples of non-radioactive carcinogens are inhaled asbestos, certain dioxins, and tobacco smoke. Although the public generally associates carcinogenicity with synthetic chemicals, it is equally likely to arise from both natural and synthetic substances. Carcinogens are not necessarily immediately toxic; thus, their effect can be insidious.

In physics, electromagnetic radiation (EMR) consists of waves of the electromagnetic (EM) field, propagating through space, carrying electromagnetic radiant energy. It includes radio waves, microwaves, infrared, (visible) light, ultraviolet, X-rays, and gamma rays. All of these waves form part of the electromagnetic spectrum.

In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or through a material medium. This includes:

Ultraviolet (UV) is a form of electromagnetic radiation with wavelength from 10 nm to 400 nm, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight, and constitutes about 10% of the total electromagnetic radiation output from the Sun. It is also produced by electric arcs and specialized lights, such as mercury-vapor lamps, tanning lamps, and black lights. Although long-wavelength ultraviolet is not considered an ionizing radiation because its photons lack the energy to ionize atoms, it can cause chemical reactions and causes many substances to glow or fluoresce. Consequently, the chemical and biological effects of UV are greater than simple heating effects, and many practical applications of UV radiation derive from its interactions with organic molecules.

A beta particle, also called beta ray or beta radiation, is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β− decay and β+ decay, which produce electrons and positrons respectively.

In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances which react with acids as originally proposed by G.-F. Rouelle in the mid-18th century.
Ionizing radiation, including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. The particles generally travel at a speed that is 99% of that of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum.

Neutron radiation is a form of ionizing radiation that presents as free neutrons. Typical phenomena are nuclear fission or nuclear fusion causing the release of free neutrons, which then react with nuclei of other atoms to form new isotopes—which, in turn, may trigger further neutron radiation. Free neutrons are unstable, decaying into a proton, an electron, plus an electron antineutrino with a mean lifetime of 887 seconds.
The free radical theory of aging (FRTA) states that organisms age because cells accumulate free radical damage over time. A free radical is any atom or molecule that has a single unpaired electron in an outer shell. While a few free radicals such as melanin are not chemically reactive, most biologically relevant free radicals are highly reactive. For most biological structures, free radical damage is closely associated with oxidative damage. Antioxidants are reducing agents, and limit oxidative damage to biological structures by passivating them from free radicals.

Reactive oxygen species (ROS) are highly reactive chemicals formed from O2. Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
A molecular lesion or point lesion is damage to the structure of a biological molecule such as DNA, RNA, or protein. This damage may result in the reduction or absence of normal function, and in rare cases the gain of a new function. Lesions in DNA may consist of breaks or other changes in chemical structure of the helix, ultimately preventing transcription. Meanwhile, lesions in proteins consist of both broken bonds and improper folding of the amino acid chain. While many nucleic acid lesions are general across DNA and RNA, some are specific to one, such as thymine dimers being found exclusively in DNA. Several cellular repair mechanisms exist, ranging from global to specific, in order to prevent lasting damage resulting from lesions.

In biochemistry and molecular genetics, an AP site, also known as an abasic site, is a location in DNA that has neither a purine nor a pyrimidine base, either spontaneously or due to DNA damage. It has been estimated that under physiological conditions 10,000 apurinic sites and 500 apyrimidinic may be generated in a cell daily.
Radiolysis is the dissociation of molecules by ionizing radiation. It is the cleavage of one or several chemical bonds resulting from exposure to high-energy flux. The radiation in this context is associated with ionizing radiation; radiolysis is therefore distinguished from, for example, photolysis of the Cl2 molecule into two Cl-radicals, where (ultraviolet or visible spectrum) light is used.
Radiation chemistry is a subdivision of nuclear chemistry which is the study of the chemical effects of radiation on matter; this is very different from radiochemistry as no radioactivity needs to be present in the material which is being chemically changed by the radiation. An example is the conversion of water into hydrogen gas and hydrogen peroxide.
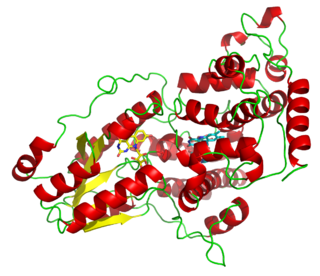
Photolyases are DNA repair enzymes that repair damage caused by exposure to ultraviolet light. These enzymes require visible light both for their own activation and for the actual DNA repair. The DNA repair mechanism involving photolyases is called photoreactivation. They mainly convert pyrimidine dimers into a normal pair of pyrimidine bases.
Radiation damage is the effect of ionizing radiation on physical objects including non-living structural materials. It can be either detrimental or beneficial for materials.
A gamma ray, also known as gamma radiation (symbol γ or ), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (30×1018 Hz), it imparts the highest photon energy. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation gamma rays based on their relatively strong penetration of matter; in 1900 he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power.
Auger therapy is a form of radiation therapy for the treatment of cancer which relies on low-energy electrons to damage cancer cells, rather than the high-energy radiation used in traditional radiation therapy. Similar to other forms of radiation therapy, Auger therapy relies on radiation-induced damage to cancer cells to arrest cell division, stop tumor growth and metastasis and kill cancerous cells. It differs from other types of radiation therapy in that electrons emitted via the Auger effect are released with low kinetic energy. In contrast to traditional α- and β-particle emitters, Auger electron emitters exhibit low cellular toxicity during transit in blood or bone marrow.
Free radical damage to DNA can occur as a result of exposure to ionizing radiation or to radiomimetic compounds. Damage to DNA as a result of free radical attack is called indirect DNA damage because the radicals formed can diffuse throughout the body and affect other organs. Malignant melanoma can be caused by indirect DNA damage because it is found in parts of the body not exposed to sunlight. DNA is vulnerable to radical attack because of the very labile hydrogens that can be abstracted and the prevalence of double bonds in the DNA bases that radicals can easily add to.
Ionizing radiation can cause biological effects which are passed on to offspring through the epigenome. The effects of radiation on cells has been found to be dependent on the dosage of the radiation, the location of the cell in regards to tissue, and whether the cell is a somatic or germ line cell. Generally, ionizing radiation appears to reduce methylation of DNA in cells.











