






Propanolamines are a class of chemical compounds, many of which are pharmaceutical drugs. They are amino alcohols that are derivatives of 1-amino-2-propanol. [1]
Contents
Propanolamines include:







Propanolamines are a class of chemical compounds, many of which are pharmaceutical drugs. They are amino alcohols that are derivatives of 1-amino-2-propanol. [1]
Propanolamines include:

Amino acids are organic compounds that contain amino (–NH2) and carboxyl (–COOH) functional groups, along with a side chain (R group) specific to each amino acid. The key elements of an amino acid are carbon (C), hydrogen (H), oxygen (O), and nitrogen (N), although other elements are found in the side chains of certain amino acids. About 500 naturally occurring amino acids are known as of 1983 (though only 20 appear in the genetic code) and can be classified in many ways. They can be classified according to the core structural functional groups' locations as alpha- (α-), beta- (β-), gamma- (γ-) or delta- (δ-) amino acids; other categories relate to polarity, pH level, and side chain group type (aliphatic, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid residues form the second-largest component (water is the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis.

The genetic code is the set of rules used by living cells to translate information encoded within genetic material into proteins. Translation is accomplished by the ribosome, which links proteinogenic amino acids in an order specified by messenger RNA (mRNA), using transfer RNA (tRNA) molecules to carry amino acids and to read the mRNA three nucleotides at a time. The genetic code is highly similar among all organisms and can be expressed in a simple table with 64 entries.

Proteins are large biomolecules or macromolecules that are comprised of one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity.
Peptides are short chains of between two and fifty amino acids, linked by peptide bonds. Chains of fewer than ten or fifteen amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.

Protein biosynthesis is a core biological process, occurring inside cells, balancing the loss of cellular proteins through the production of new proteins. Proteins perform a number of critical functions as enzymes, structural proteins or hormones. Protein synthesis is a very similar process for both prokaryotes and eukaryotes but there are some distinct differences.

Phenylalanine is an essential α-amino acid with the formula C
9H
11NO
2. It can be viewed as a benzyl group substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino acid is classified as neutral, and nonpolar because of the inert and hydrophobic nature of the benzyl side chain. The L-isomer is used to biochemically form proteins, coded for by DNA. Phenylalanine is a precursor for tyrosine, the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the skin pigment melanin. It is encoded by the codons UUU and UUC.

Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula HOOC-CH-(NH2)-CH2-SH. The thiol side chain in cysteine often participates in enzymatic reactions, as a nucleophile. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. When used as a food additive, it has the E number E920. It is encoded by the codons UGU and UGC.

Alanine (symbol Ala or A) is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG).
An essential amino acid, or indispensable amino acid, is an amino acid that cannot be synthesized from scratch by the organism fast enough to supply its demand, and must therefore come from the diet. Of the 21 amino acids common to all life forms, the nine amino acids humans cannot synthesize are phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, and histidine.

In molecular biology and genetics, translation is the process in which ribosomes in the cytoplasm or endoplasmic reticulum synthesize proteins after the process of transcription of DNA to RNA in the cell's nucleus. The entire process is called gene expression.
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to another carboxylic group in a protein to make it a chain, but since the end amino acid of a protein is only connected at the carboxy- end, the remaining free amine group is called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction (because when a protein is translated from messenger RNA, it is created from N-terminus to C-terminus - amino acids are added to the carboxyl end).

Ethanolamine (2-aminoethanol, monoethanolamine, ETA, or MEA) is an organic chemical compound with the formula HOCH2CH2NH2 (C2H7NO). The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid with an odor reminiscent of ammonia. Its derivatives are widespread in nature; e.g., lipids, as precursor of a variety of N-acylethanolamines (NAEs), that modulate several animal and plant physiological processes such as seed germination, plant–pathogen interactions, chloroplast development and flowering, as well as precursor, combined with arachidonic acid (C20H32O2; 20:4, ω-6), to form the endocannabinoid Anandamide (AEA: C22H37NO2; 20:4, ω-6).

N-Methylethanolamine is an alkanolamine with the formula CH3NHCH2CH2OH. It is flammable, corrosive, colorless, viscous liquid. It is an intermediate in the biosynthesis of choline.

Dimethylethanolamine (DMAE or DMEA) is an organic compound with the formula (CH3)2NCH2CH2OH. It is bifunctional, containing both a tertiary amine and primary alcohol functional groups. It is a colorless viscous liquid. It is used in skin care products. It is prepared by the ethoxylation of dimethylamine.
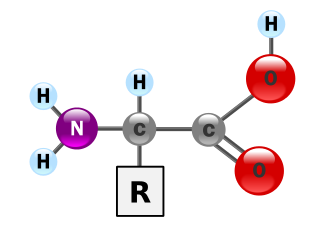
Proteins are essential nutrients for the human body. They are one of the building blocks of body tissue and can also serve as a fuel source. As a fuel, proteins provide as much energy density as carbohydrates: 4 kcal per gram; in contrast, lipids provide 9 kcal per gram. The most important aspect and defining characteristic of protein from a nutritional standpoint is its amino acid composition.
Alkanolamines are chemical compounds that contain both hydroxyl (-OH) and amino (-NH2, -NHR, and -NR2) functional groups on an alkane backbone. The term alkanolamine is a broad class term that is sometimes used as a subclassification.

Methyl diethanolamine, also known as N-methyl diethanolamine and more commonly as MDEA, is the organic compound with the formula CH3N(C2H4OH)2. It is a colorless liquid with an ammonia odor. It is miscible with water, ethanol and benzene. A tertiary amine, it is widely used as a sweetening agent in chemical, oil refinery, syngas production and natural gas.

Flestolol is a short-acting beta adrenergic receptor antagonist.

Enciprazine, is an anxiolytic and antipsychotic of the phenylpiperazine class which was never marketed. It shows high affinity for the α1-adrenergic receptor and 5-HT1A receptor, among other sites. The drug was initially anticipated to produce ortho-methoxyphenylpiperazine (oMeOPP), a serotonin receptor agonist with high affinity for the 5-HT1A receptor, as a significant active metabolite, but subsequent research found this not to be the case.
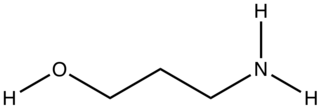
3-Amino-1-propanol is the organic compound with the formula HOCH2CH2CH2NH2. A colorless liquid, the compound is one of the simplest aminopropanols.