Related Research Articles
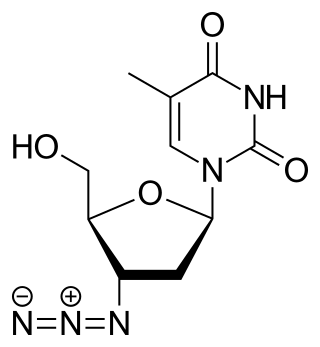
Zidovudine (ZDV), also known as azidothymidine (AZT), is an antiretroviral medication used to prevent and treat HIV/AIDS. It is generally recommended for use in combination with other antiretrovirals. It may be used to prevent mother-to-child spread during birth or after a needlestick injury or other potential exposure. It is sold both by itself and together as lamivudine/zidovudine and abacavir/lamivudine/zidovudine. It can be used by mouth or by slow injection into a vein.
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs as a strategy to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multiple drugs that act on different viral targets is known as highly active antiretroviral therapy (HAART). HAART decreases the patient's total burden of HIV, maintains function of the immune system, and prevents opportunistic infections that often lead to death. HAART also prevents the transmission of HIV between serodiscordant same sex and opposite sex partners so long as the HIV-positive partner maintains an undetectable viral load.
The spread of HIV/AIDS has affected millions of people worldwide; AIDS is considered a pandemic. The World Health Organization (WHO) estimated that in 2016 there were 36.7 million people worldwide living with HIV/AIDS, with 1.8 million new HIV infections per year and 1 million deaths due to AIDS. Misconceptions about HIV and AIDS arise from several different sources, from simple ignorance and misunderstandings about scientific knowledge regarding HIV infections and the cause of AIDS to misinformation propagated by individuals and groups with ideological stances that deny a causative relationship between HIV infection and the development of AIDS. Below is a list and explanations of some common misconceptions and their rebuttals.
Protease inhibitors (PIs) are medications that act by interfering with enzymes that cleave proteins. Some of the most well known are antiviral drugs widely used to treat HIV/AIDS and hepatitis C. These protease inhibitors prevent viral replication by selectively binding to viral proteases and blocking proteolytic cleavage of protein precursors that are necessary for the production of infectious viral particles.

Nevirapine (NVP), sold under the brand name Viramune among others, is a medication used to treat and prevent HIV/AIDS, specifically HIV-1. It is generally recommended for use with other antiretroviral medications. It may be used to prevent mother to child spread during birth but is not recommended following other exposures. It is taken by mouth.
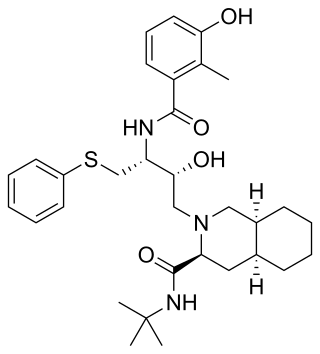
Nelfinavir, sold under the brand name Viracept, is an antiretroviral medication used in the treatment of HIV/AIDS. Nelfinavir belongs to the class of drugs known as protease inhibitors (PIs) and like other PIs is almost always used in combination with other antiretroviral drugs.
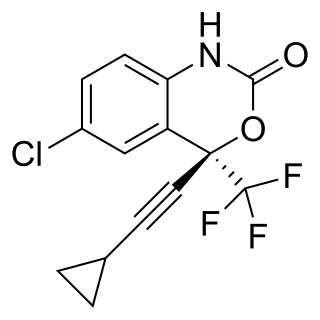
Efavirenz (EFV), sold under the brand names Sustiva among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. It is sold both by itself and in combination as efavirenz/emtricitabine/tenofovir. It is taken by mouth.

Saquinavir, sold under the brand name Invirase among others, is an antiretroviral medication used together with other medications to treat or prevent HIV/AIDS. Typically it is used with ritonavir or lopinavir/ritonavir to increase its effect. It is taken by mouth.
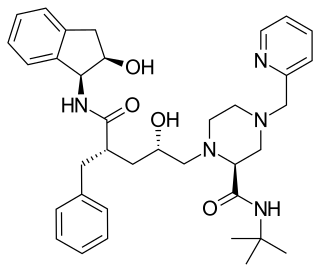
Indinavir is a protease inhibitor used as a component of highly active antiretroviral therapy to treat HIV/AIDS. It is soluble white powder administered orally in combination with other antiviral drugs. The drug prevents protease from functioning normally. Consequently, HIV viruses cannot reproduce, causing a decrease in the viral load. Commercially sold indinavir is indinavir anhydrous, which is indinavir with an additional amine in the hydroxyethylene backbone. This enhances its solubility and oral bioavailability, making it easier for users to intake. It was synthetically produced for the purpose of inhibiting the protease in the HIV virus.

Darunavir (DRV), sold under the brand name Prezista among others, is an antiretroviral medication used to treat and prevent HIV/AIDS. It is generally recommended for use with other antiretrovirals. It is often used with low doses of ritonavir or cobicistat to increase darunavir levels. It may be used for prevention after a needlestick injury or other potential exposure. It is taken by mouth once to twice a day.

Raltegravir, sold under the brand name Isentress, is an antiretroviral medication used, together with other medication, to treat HIV/AIDS. It may also be used, as part of post exposure prophylaxis, to prevent HIV infection following potential exposure. It is taken by mouth.
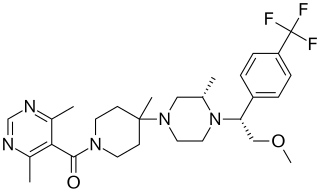
Vicriviroc, previously named SCH 417690 and SCH-D, is a pyrimidine CCR5 entry inhibitor of HIV-1. It was developed by the pharmaceutical company Schering-Plough. Merck decided to not pursue regulatory approval for use in treatment-experienced patients because the drug did not meet primary efficacy endpoints in late stage trials. Clinical trials continue in patients previously untreated for HIV.

Rilpivirine, sold under the brand names Edurant and Rekambys, is a medication, developed by Tibotec, used for the treatment of HIV/AIDS. It is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) with higher potency, longer half-life and reduced side-effect profile compared with older NNRTIs such as efavirenz.
Many major physiological processes depend on regulation of proteolytic enzyme activity and there can be dramatic consequences when equilibrium between an enzyme and its substrates is disturbed. In this prospective, the discovery of small-molecule ligands, like protease inhibitors, that can modulate catalytic activities has an enormous therapeutic effect. Hence, inhibition of the HIV protease is one of the most important approaches for the therapeutic intervention in HIV infection and their development is regarded as major success of structure-based drug design. They are highly effective against HIV and have, since the 1990s, been a key component of anti-retroviral therapies for HIV/AIDS.
HIV drug resistance occurs when microevolution causes virions to become tolerant to antiretroviral treatments (ART). ART can be used to successfully manage HIV infection, but a number of factors can contribute to the virus mutating and becoming resistant. Drug resistance occurs as bacterial or viral populations evolve to no longer respond to medications that previously worked. In the case of HIV, there have been recognized cases of treatment resistant strains since 1989, with drug resistance being a major contributor to treatment failure. While global incidence varies greatly from region to region, there has been a general increase in overall HIV drug resistance. The two main types of resistance, primary and induced, differ mostly in causation, with the biggest cause of resistance being a lack of adherence to the specific details of treatment. These newly created resistant strains of HIV pose a public health issue as they infect a growing number of people because they are harder to treat, and can be spread to other individuals. For this reason, the reaction to the growing number of cases of resistant HIV strains has mostly been to try to increase access to treatment and implement other measures to make sure people stay in care, as well as to look into the development of a HIV vaccine or cure.

Lopinavir/ritonavir (LPV/r), sold under the brand name Kaletra among others, is a fixed-dose combination antiretroviral medication for the treatment and prevention of HIV/AIDS. It combines lopinavir with a low dose of ritonavir. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. It is taken by mouth as a tablet, capsule, or solution.

Simeprevir, sold under the trade names Olysio among others, is a medication used in combination with other medications for the treatment of hepatitis C. It is specifically used for hepatitis C genotype 1 and 4. Medications it is used with include sofosbuvir or ribavirin and peginterferon-alfa. Cure rates are in 80s to 90s percent. It may be used in those who also have HIV/AIDS. It is taken by mouth once daily for typically 12 weeks.
HIV/AIDS research includes all medical research that attempts to prevent, treat, or cure HIV/AIDS, as well as fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
HIV in pregnancy is the presence of an HIV/AIDS infection in a woman while she is pregnant. There is a risk of HIV transmission from mother to child in three primary situations: pregnancy, childbirth, and while breastfeeding. This topic is important because the risk of viral transmission can be significantly reduced with appropriate medical intervention, and without treatment HIV/AIDS can cause significant illness and death in both the mother and child. This is exemplified by data from The Centers for Disease Control (CDC): In the United States and Puerto Rico between the years of 2014–2017, where prenatal care is generally accessible, there were 10,257 infants in the United States and Puerto Rico who were exposed to a maternal HIV infection in utero who did not become infected and 244 exposed infants who did become infected.
Treatment as prevention (TasP) is a concept in public health that promotes treatment as a way to prevent and reduce the likelihood of HIV illness, death and transmission from an infected individual to others. Expanding access to earlier HIV diagnosis and treatment as a means to address the global epidemic by preventing illness, death and transmission was first proposed in 2000 by Garnett et al. The term is often used to talk about treating people that are currently living with human immunodeficiency virus (HIV) and acquired immune deficiency syndrome (AIDS) to prevent illness, death and transmission. Although some experts narrow this to only include preventing infections, treatment prevents illnesses such as tuberculosis and has been shown to prevent death. The dual impact on well-being and its 100% effectiveness in reducing transmission makes TasP the most important element in the HIV prevention toolkit. In relation to HIV, antiretroviral therapy (ART) is a three or more drug combination therapy that is used to decrease the viral load, or the measured amount of virus, in an infected individual. Such medications are used as a preventative for infected individuals to not only spread the HIV virus to their negative partners but also improve their current health to increase their lifespans. Other names for ART include highly active antiretroviral therapy (HAART), combination antiretroviral therapy (cART), triple therapy and triple drug cocktail. When taken correctly, ART is able to diminish the presence of the HIV virus in the bodily fluids of an infected person to a level of undetectability. Undetectability ensures that infection does not necessarily have an effect on a person's general health, and that there is no longer a risk of passing along HIV to others. Consistent adherence to an ARV regimen, monitoring, and testing are essential for continued confirmed viral suppression. Treatment as prevention rose to great prominence in 2011, as part of the HPTN 052 study, which shed light on the benefits of early treatment for HIV positive individuals.
References
- ↑ Corales RB, Shrestha NK, Taege AJ, et al. (2001). "Protease-sparing regimen in a real-life practice with naïve patients: an equal opportunity approach?". HIV Clin Trials. 2 (1): 17–21. doi:10.1310/2V0B-HDWC-AGWR-H56M. PMID 11590510. S2CID 26663673.
- ↑ Van der Linden D, Hainaut M, Goetghebuer T, et al. (April 2007). "Effectiveness of early initiation of protease inhibitor-sparing antiretroviral regimen in human immunodeficiency virus-1 vertically infected infants". Pediatr. Infect. Dis. J. 26 (4): 359–61. doi:10.1097/01.inf.0000258626.34984.eb. hdl:2078/119175. PMID 17414406. S2CID 74548567.