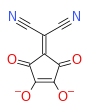
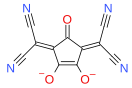
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a diprotic organic acid with the chemical formula C4O2(OH)2.
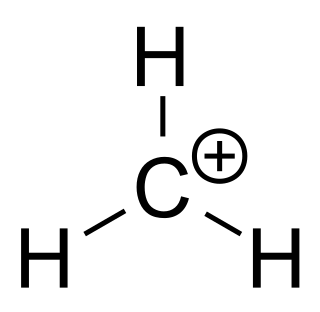
A carbenium ion is a positive ion with the structure RR′R″C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. But IUPAC confuses coordination number with valence, incorrectly considering carbon in carbenium as trivalent.

In chemistry, an oxocarbon or oxide of carbon is a chemical compound consisting only of carbon and oxygen. The simplest and most common oxocarbons are carbon monoxide (CO) and carbon dioxide. Many other stable or metastable oxides of carbon are known, but they are rarely encountered, such as carbon suboxide and mellitic anhydride.
Thiocarbonate describes a family of anions with the general chemical formula CS
3−xO2−
x (x = 0, 1, or 2):

An oxocarbeniumion is a chemical species characterized by a central sp2-hybridized carbon, an oxygen substituent, and an overall positive charge that is delocalized between the central carbon and oxygen atoms. An oxocarbenium ion is represented by two limiting resonance structures, one in the form of a carbenium ion with the positive charge on carbon and the other in the form of an oxonium species with the formal charge on oxygen. As a resonance hybrid, the true structure falls between the two. Compared to neutral carbonyl compounds like ketones or esters, the carbenium ion form is a larger contributor to the structure. They are common reactive intermediates in the hydrolysis of glycosidic bonds, and are a commonly used strategy for chemical glycosylation. These ions have since been proposed as reactive intermediates in a wide range of chemical transformations, and have been utilized in the total synthesis of several natural products. In addition, they commonly appear in mechanisms of enzyme-catalyzed biosynthesis and hydrolysis of carbohydrates in nature. Anthocyanins are natural flavylium dyes, which are stabilized oxocarbenium compounds. Anthocyanins are responsible for the colors of a wide variety of common flowers such as pansies and edible plants such as eggplant and blueberry.
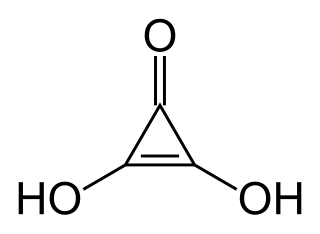
Deltic acid (also known as dihydroxycyclopropenone or trianglic acid) is a chemical substance with the chemical formula C3O(OH)2. It can be viewed as a ketone and double enol of cyclopropene. At room temperature, it is a stable white solid, soluble in diethyl ether, that decomposes (sometimes explosively) between 140 °C and 180 °C, and reacts slowly with water.

Croconic acid is a chemical compound with formula C5H2O5 or (C=O)3(COH)2. It has a cyclopentene backbone with two hydroxyl groups adjacent to the double bond and three ketone groups on the remaining carbon atoms. It is sensitive to light, soluble in water and ethanol and forms yellow crystals that decompose at 212 °C.
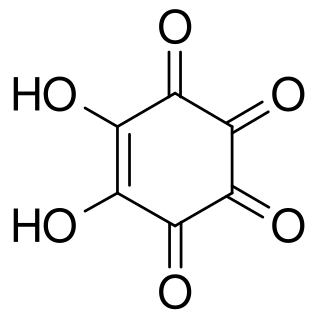
Rhodizonic acid is a chemical compound with formula C6H2O6 or (CO)4(COH)2. It can be seen as a twofold enol and fourfold ketone of cyclohexene, more precisely 5,6-dihydroxycyclohex-5-ene-1,2,3,4-tetrone.
In chemistry, an oxocarbon anion is a negative ion consisting solely of carbon and oxygen atoms, and therefore having the general formula C
xOn−
y for some integers x, y, and n.

A dicarbonate, also known as a pyrocarbonate, is a chemical containing the divalent −O−C(=O)−O−C(=O)−O− or −C2O5− functional group, which consists of two carbonate groups sharing an oxygen atom. These compounds can be viewed as derivatives of the hypothetical compound dicarbonic acid, HO−C(=O)−O−C(=O)−OH or H2C2O5. Two important organic compounds containing this group are dimethyl dicarbonate H3C−C2O5−CH3 and di-tert-butyl dicarbonate(H3C−)3C−C2O5−C(−CH3)3.

Croconate violet or 1,3-bis(dicyanomethylene)croconate is a divalent anion with chemical formula C
11N
4O2−
3 or ((N≡C−)2C=)2(C5O3)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of two oxygen atoms by dicyanomethylene groups =C(−C≡N)2. Its systematic name is 3,5-bis(dicyanomethylene)-1,2,4-trionate. The term croconate violet as a dye name specifically refers to the dipotassium salt K
2C
11N
4O
3.

1,2-Bis(dicyanomethylene)squarate is a divalent anion with chemical formula C
10N
4O2−
2 or ((N≡C−)2C=)2(C4O2)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the squarate oxocarbon anion C
4O2−
4 through the replacement of two adjacent oxygen atoms by dicyanomethylene groups =C(−C≡N)2.

1,3-Bis(dicyanomethylene)squarate is a divalent anion with chemical formula C
10N
4O2−
2 or ((N≡C−)2C=)2(C4O2)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the squarate oxocarbon anion C
4O2−
4 through the replacement of two opposite oxygen atoms by dicyanomethylene groups =C(−C≡N)2.
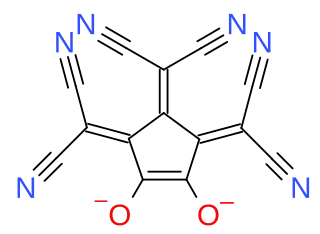
Croconate blue or 1,2,3-tris(dicyanomethylene)croconate is a divalent anion with chemical formula C
14N
6O2−
2 or ((N≡C−)2C=)3(C5O2)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of three oxygen atoms by dicyanomethylene groups =C(−C≡N)2. The term Croconate Blue as a dye name specifically refers to the dipotassium salt K
2C
14N
6O
2.

2-(Dicyanomethylene)croconate is a divalent anion with chemical formula C
8N
2O2−
4 or ((N≡C−)2C=)(C5O4)2−. It is one of the pseudo-oxocarbon anions, as it can be described as a derivative of the croconate oxocarbon anion C
5O2−
5 through the replacement of one oxygen atom by a dicyanomethylene group =C(−C≡N)2.
Alexander Johann Fatiadi was a chemist.
Boroles represent a class of molecules known as metalloles, which are heterocyclic 5-membered rings. As such, they can be viewed as structural analogs of cyclopentadiene, pyrrole or furan, with boron replacing a carbon, nitrogen and oxygen atom respectively. They are isoelectronic with the cyclopentadienyl cation C5H+5(Cp+) and comprise four π electrons. Although Hückel's rule cannot be strictly applied to borole, it is considered to be antiaromatic due to having 4 π electrons. As a result, boroles exhibit unique electronic properties not found in other metalloles.
The carbonite ion is the double ionized ion of dihydroxymethylidene, with the chemical formula: CO2−
2. Alkali metal salts, Li
2CO
2, K
2CO
2, Rb
2CO
2 and Cs
2CO
2, have been observed at 15 K. Interestingly, sodium does not form a carbonite. Due to the lone pair on the carbon atom, salts of the carbonite ion would be protonated to form formate and formic acid, rather than the carbene.

A metaborate is a borate anion consisting of boron and oxygen, with empirical formula BO−2. Metaborate also refers to any salt or ester of such anion. Metaborate is one of the boron's oxyanions. Metaborates can be monomeric, oligomeric or polymeric.
The phosphaethynolate anion, also referred to as PCO, is the phosphorus-containing analogue of the cyanate anion with the chemical formula [PCO]− or [OCP]−. The anion has a linear geometry and is commonly isolated as a salt. When used as a ligand, the phosphaethynolate anion is ambidentate in nature meaning it forms complexes by coordinating via either the phosphorus or oxygen atoms. This versatile character of the anion has allowed it to be incorporated into many transition metal and actinide complexes but now the focus of the research around phosphaethynolate has turned to utilising the anion as a synthetic building block to organophosphanes.