Related Research Articles
Henipavirus is a genus of negative-strand RNA viruses in the family Paramyxoviridae, order Mononegavirales containing six established species, and numerous others still under study. Henipaviruses are naturally harboured by several species of small mammals, notably pteropid fruit bats, microbats of several species, and shrews. Henipaviruses are characterised by long genomes and a wide host range. Their recent emergence as zoonotic pathogens capable of causing illness and death in domestic animals and humans is a cause of concern.
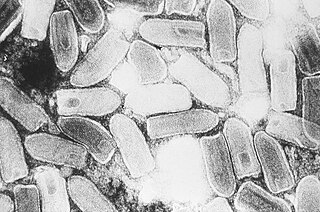
Indiana vesiculovirus, formerly Vesicular stomatitis Indiana virus is a virus in the family Rhabdoviridae; the well-known Rabies lyssavirus belongs to the same family. VSIV can infect insects, cattle, horses and pigs. It has particular importance to farmers in certain regions of the world where it infects cattle. This is because its clinical presentation is identical to the very important foot and mouth disease virus.

The genus Ebolavirus is a virological taxon included in the family Filoviridae, order Mononegavirales. The members of this genus are called ebolaviruses, and encode their genome in the form of single-stranded negative-sense RNA. The six known virus species are named for the region where each was originally identified: Bundibugyo ebolavirus, Reston ebolavirus, Sudan ebolavirus, Taï Forest ebolavirus, Zaire ebolavirus, and Bombali ebolavirus. The last is the most recent species to be named and was isolated from Angolan free-tailed bats in Sierra Leone. Each species of the genus Ebolavirus has one member virus, and four of these cause Ebola virus disease (EVD) in humans, a type of hemorrhagic fever having a very high case fatality rate. The Reston virus has caused EVD in other primates. Zaire ebolavirus has the highest mortality rate of the ebolaviruses and is responsible for the largest number of outbreaks of the six known species of the genus, including the 1976 Zaire outbreak and the outbreak with the most deaths (2014).
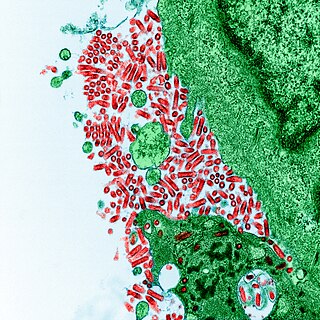
Rabies virus, scientific name Rabies lyssavirus, is a neurotropic virus that causes rabies in animals, including humans. Rabies transmission can occur through the saliva of animals and less commonly through contact with human saliva. Rabies lyssavirus, like many rhabdoviruses, has an extremely wide host range. In the wild it has been found infecting many mammalian species, while in the laboratory it has been found that birds can be infected, as well as cell cultures from mammals, birds, reptiles and insects. Rabies is reported in more than 150 countries and on all continents except Antarctica. The main burden of disease is reported in Asia and Africa, but some cases have been reported also in Europe in the past 10 years, especially in returning travellers.
Virus-like particles (VLPs) are molecules that closely resemble viruses, but are non-infectious because they contain no viral genetic material. They can be naturally occurring or synthesized through the individual expression of viral structural proteins, which can then self assemble into the virus-like structure. Combinations of structural capsid proteins from different viruses can be used to create recombinant VLPs. Both in-vivo assembly and in-vitro assembly have been successfully shown to form virus-like particles. VLPs derived from the Hepatitis B virus (HBV) and composed of the small HBV derived surface antigen (HBsAg) were described in 1968 from patient sera. VLPs have been produced from components of a wide variety of virus families including Parvoviridae, Retroviridae, Flaviviridae, Paramyxoviridae and bacteriophages. VLPs can be produced in multiple cell culture systems including bacteria, mammalian cell lines, insect cell lines, yeast and plant cells.

Baculoviridae is a family of viruses. Arthropods, among the most studied being Lepidoptera, Hymenoptera and Diptera, serve as natural hosts. Currently, 85 species are placed in this family, assigned to four genera.
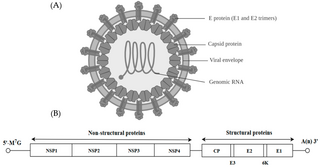
Alphavirus is a genus of RNA viruses, the sole genus in the Togaviridae family. Alphaviruses belong to group IV of the Baltimore classification of viruses, with a positive-sense, single-stranded RNA genome. There are 32 alphaviruses, which infect various vertebrates such as humans, rodents, fish, birds, and larger mammals such as horses, as well as invertebrates. Alphaviruses that could infect both vertebrates and arthropods are referred dual-host alphaviruses, while insect-specific alphaviruses such as Eilat virus and Yada yada virus are restricted to their competent arthropod vector. Transmission between species and individuals occurs mainly via mosquitoes, making the alphaviruses a member of the collection of arboviruses – or arthropod-borne viruses. Alphavirus particles are enveloped, have a 70 nm diameter, tend to be spherical, and have a 40 nm isometric nucleocapsid.

A viral envelope is the outermost layer of many types of viruses. It protects the genetic material in their life cycle when traveling between host cells. Not all viruses have envelopes. A viral envelope protein or E protein is a protein in the envelope, which may be acquired by the capsid from an infected host cell.
Mason-Pfizer monkey virus (M-PMV), formerly Simian retrovirus (SRV), is a species of retroviruses that usually infect and cause a fatal immune deficiency in Asian macaques. The ssRNA virus appears sporadically in mammary carcinoma of captive macaques at breeding facilities which expected as the natural host, but the prevalence of this virus in feral macaques remains unknown. M-PMV was transmitted naturally by virus-containing body fluids, via biting, scratching, grooming, and fighting. Cross contaminated instruments or equipment (fomite) can also spread this virus among animals.

The family of vesiculovirus matrix proteins consists of several matrix proteins of the vesicular stomatitis virus, also known as VSIV or VSV. The matrix (M) protein of the virus causes many of the cytopathic effects of VSV, including an inhibition of host gene expression and the induction of cell rounding. It has been shown that M protein also induces apoptosis in the absence of other viral components. It is thought that the activation of apoptotic pathways causes the inhibition of host gene expression and cell rounding by M protein.

Lentiviral vectors in gene therapy is a method by which genes can be inserted, modified, or deleted in organisms using lentiviruses.

Zaire ebolavirus, more commonly known as Ebola virus, is one of six known species within the genus Ebolavirus. Four of the six known ebolaviruses, including EBOV, cause a severe and often fatal hemorrhagic fever in humans and other mammals, known as Ebola virus disease (EVD). Ebola virus has caused the majority of human deaths from EVD, and was the cause of the 2013–2016 epidemic in western Africa, which resulted in at least 28,646 suspected cases and 11,323 confirmed deaths.

Recombinant vesicular stomatitis virus–Zaire Ebola virus (rVSV-ZEBOV), also known as Ebola Zaire vaccine live and sold under the brand name Ervebo, is an Ebola vaccine for adults that prevents Ebola caused by the Zaire ebolavirus. When used in ring vaccination, rVSV-ZEBOV has shown a high level of protection. Around half the people given the vaccine have mild to moderate adverse effects that include headache, fatigue, and muscle pain.

Ebola vaccines are vaccines either approved or in development to prevent Ebola. As of 2022, there are only vaccines against the Zaire ebolavirus. The first vaccine to be approved in the United States was rVSV-ZEBOV in December 2019. It had been used extensively in the Kivu Ebola epidemic under a compassionate use protocol. During the early 21st century, several vaccine candidates displayed efficacy to protect nonhuman primates against lethal infection.
David Mahan Knipe is the Higgins Professor of Microbiology and Molecular Genetics in the Department of Microbiology at the Harvard Medical School in Boston, Massachusetts and co-chief editor of the reference book Fields Virology. He returned to the Chair of the Program in Virology at Harvard Medical School in 2019, having previously held the position from 2004 through 2016 and served as interim Co-Chair of the Microbiology and Immunobiology Department from 2016 through 2018.

Sean Whelan is a British-American virologist. He is known for identifying the cellular protein used as a receptor by Ebola virus, for defining the entry pathway that rabies virus uses to enter neurons, and for identifying the ribosome as a possible target for antiviral drugs. In July 2019, he was announced as the new Chair of the Department of Molecular Microbiology at Washington University School of Medicine in St Louis, Missouri. In February 2020, Whelan was recognized as the LGBTQ+ Scientist of the Year 2020 by the National Organization of Gay and Lesbian Scientists and Technical Professionals.
GamEvac-Combi is a heterologous VSV- and Ad5-vectored Ebola vaccine. There is also a version called GamEvac which is a homologous Ad5-vectored vaccine. GamEvac-Combi was developed by Gamaleya Research Institute of Epidemiology and Microbiology. As of 2015 the vaccine has been licensed in Russia for emergency use, on the basis of Phase 1 and Phase 2 clinical trials.

Reporter virus particles (RVPs) are replication-incompetent virus particles engineered to express one or more reporter genes upon infecting susceptible cells. Since the RVP genome lacks genes essential for viral replication, RVPs are capable of only a single round of infection. Thus they are safe to work with under BSL-2 conditions, enabling the study of highly pathogenic viruses using standard laboratory facilities. Expression of a reporter such as luciferase can provide a quantitative readout of infection. With proper design and quality control, RVPs remain stable under common assay conditions and yield reproducible results that correlate with those obtained from live virus. These qualities make RVPs a safer and faster alternative to plaque assays, and especially well-suited for high-throughput applications. RVPs offer flexibility for different uses, as they are antigenically identical to wild-type virus, and can be engineered with various proteins or express mutant envelopes to study infectivity or antigenicity.

A viral vector vaccine is a vaccine that uses a viral vector to deliver genetic material (DNA) that can be transcribed by the recipient's host cells as mRNA coding for a desired protein, or antigen, to elicit an immune response. As of April 2021, six viral vector vaccines, four COVID-19 vaccines and two Ebola vaccines, have been authorized for use in humans.
Endothelial cell tropism or endotheliotropism is a type of tissue tropism or host tropism that characterizes an pathogen's ability to recognize and infect an endothelial cell. Pathogens, such as viruses, can target a specific tissue type or multiple tissue types. Like other cells, the endothelial cell possesses several features that supports a productive viral infection a cell including, cell surface receptors, immune responses, and other virulence factors. Endothelial cells are found in various tissue types such as in the capillaries, veins, and arteries in the human body. As endothelial cells line these blood vessels and critical networks that extend access to various human organ systems, the virus entry into these cells can be detrimental to virus spread across the host system and affect clinical course of disease. Understanding the mechanisms of how viruses attach, enter, and control endothelial functions and host responses inform infectious disease understanding and medical countermeasures.
References
- ↑ Example for the development of pseudotype retroviral vectors in a work group of MHH Archived 2009-11-08 at the Wayback Machine
- 1 2 Nie, Jianhui; Liu, Lin; Wang, Qing; Chen, Ruifeng; Ning, Tingting; Liu, Qiang; Huang, Weijin; Wang, Youchun (2019-02-19). "Nipah pseudovirus system enables evaluation of vaccines in vitro and in vivo using non-BSL-4 facilities". Emerging Microbes & Infections. 8 (1): 272–281. doi:10.1080/22221751.2019.1571871. ISSN 2222-1751. PMC 6455126 . PMID 30866781.
- 1 2 Racine, Trina; Kobinger, Gary P.; Arts, Eric J. (2017-09-12). "Development of an HIV vaccine using a vesicular stomatitis virus vector expressing designer HIV-1 envelope glycoproteins to enhance humoral responses". AIDS Research and Therapy. 14 (1): 55. doi: 10.1186/s12981-017-0179-2 . ISSN 1742-6405. PMC 5594459 . PMID 28893277.
- 1 2 Moeschler, Sarah; Locher, Samira; Conzelmann, Karl-Klaus; Krämer, Beate; Zimmer, Gert (2016-09-16). "Quantification of Lyssavirus-Neutralizing Antibodies Using Vesicular Stomatitis Virus Pseudotype Particles". Viruses. 8 (9): 254. doi: 10.3390/v8090254 . ISSN 1999-4915. PMC 5035968 . PMID 27649230.
- ↑ Kapadia, Sagar U.; Simon, Ian D.; Rose, John K. (2008-06-20). "SARS vaccine based on a replication-defective recombinant vesicular stomatitis virus is more potent than one based on a replication-competent vector". Virology. 376 (1): 165–172. doi:10.1016/j.virol.2008.03.002. ISSN 0042-6822. PMC 7103385 . PMID 18396306.
- ↑ Salata, Cristiano; Calistri, Arianna; Alvisi, Gualtiero; Celestino, Michele; Parolin, Cristina; Palù, Giorgio (2019-03-19). "Ebola Virus Entry: From Molecular Characterization to Drug Discovery". Viruses. 11 (3): 274. doi: 10.3390/v11030274 . ISSN 1999-4915. PMC 6466262 . PMID 30893774.
- ↑ Johnson, Marc C.; Lyddon, Terri D.; Suarez, Reinier; Salcedo, Braxton; LePique, Mary; Graham, Maddie; Ricana, Clifton; Robinson, Carolyn; Ritter, Detlef G. (2020-10-14). "Optimized Pseudotyping Conditions for the SARS-COV-2 Spike Glycoprotein". Journal of Virology. 94 (21). doi: 10.1128/JVI.01062-20 . ISSN 0022-538X. PMC 7565639 . PMID 32788194.
- ↑ Carnell, George William; Ferrara, Francesca; Grehan, Keith; Thompson, Craig Peter; Temperton, Nigel James (2015-04-29). "Pseudotype-Based Neutralization Assays for Influenza: A Systematic Analysis". Frontiers in Immunology. 6: 161. doi: 10.3389/fimmu.2015.00161 . ISSN 1664-3224. PMC 4413832 . PMID 25972865.