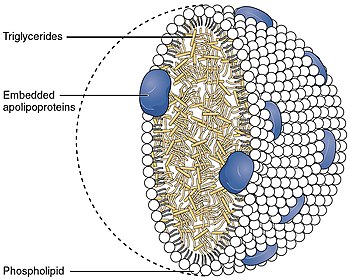
The low-density lipoprotein receptor (LDL-R) is a mosaic protein of 839 amino acids (after removal of 21-amino acid signal peptide) [5] that mediates the endocytosis of cholesterol-rich low-density lipoprotein (LDL). It is a cell-surface receptor that recognizes apolipoprotein B100 (ApoB100), which is embedded in the outer phospholipid layer of very low-density lipoprotein (VLDL), their remnants—i.e. intermediate-density lipoprotein (IDL), and LDL particles. The receptor also recognizes apolipoprotein E (ApoE) which is found in chylomicron remnants and IDL. In humans, the LDL receptor protein is encoded by the LDLR gene on chromosome 19. [6] [7] [8] It belongs to the low density lipoprotein receptor gene family. [9] It is most significantly expressed in bronchial epithelial cells and adrenal gland and cortex tissue. [10]
Michael S. Brown and Joseph L. Goldstein were awarded the 1985 Nobel Prize in Physiology or Medicine for their identification of LDL-R [11] and its relation to cholesterol metabolism and familial hypercholesterolemia. [12] Disruption of LDL-R can lead to higher LDL-cholesterol as well as increasing the risk of related diseases. Individuals with disruptive mutations (defined as nonsense, splice site, or indel frameshift) in LDLR have an average LDL-cholesterol of 279 mg/dL, compared with 135 mg/dL for individuals with neither disruptive nor deleterious mutations. Disruptive mutations were 13 times more common in individuals with early-onset myocardial infarction or coronary artery disease than in individuals without either disease. [13]