Cholesterol is the principal sterol of all higher animals, distributed in body tissues, especially the brain and spinal cord, and in animal fats and oils.
High-density lipoprotein (HDL) is one of the five major groups of lipoproteins. Lipoproteins are complex particles composed of multiple proteins which transport all fat molecules (lipids) around the body within the water outside cells. They are typically composed of 80–100 proteins per particle. HDL particles enlarge while circulating in the blood, aggregating more fat molecules and transporting up to hundreds of fat molecules per particle.

Low-density lipoprotein (LDL) is one of the five major groups of lipoprotein that transport all fat molecules around the body in extracellular water. These groups, from least dense to most dense, are chylomicrons, very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL) and high-density lipoprotein (HDL). LDL delivers fat molecules to cells. LDL has been associated with the progression of atherosclerosis.

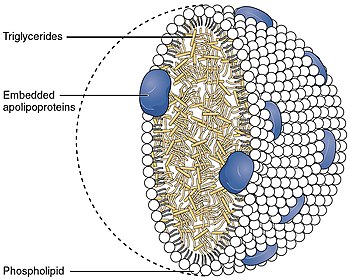
A lipoprotein is a biochemical assembly whose primary function is to transport hydrophobic lipid molecules in water, as in blood plasma or other extracellular fluids. They consist of a triglyceride and cholesterol center, surrounded by a phospholipid outer shell, with the hydrophilic portions oriented outward toward the surrounding water and lipophilic portions oriented inward toward the lipid center. A special kind of protein, called apolipoprotein, is embedded in the outer shell, both stabilising the complex and giving it a functional identity that determines its role.
Very-low-density lipoprotein (VLDL), density relative to extracellular water, is a type of lipoprotein made by the liver. VLDL is one of the five major groups of lipoproteins that enable fats and cholesterol to move within the water-based solution of the bloodstream. VLDL is assembled in the liver from triglycerides, cholesterol, and apolipoproteins. VLDL is converted in the bloodstream to low-density lipoprotein (LDL) and intermediate-density lipoprotein (IDL). VLDL particles have a diameter of 30–80 nanometers (nm). VLDL transports endogenous products, whereas chylomicrons transport exogenous (dietary) products. In the early 2010s both the lipid composition and protein composition of this lipoprotein were characterised in great detail.
Hyperlipidemia is abnormally high levels of any or all lipids or lipoproteins in the blood. The term hyperlipidemia refers to the laboratory finding itself and is also used as an umbrella term covering any of various acquired or genetic disorders that result in that finding. Hyperlipidemia represents a subset of dyslipidemia and a superset of hypercholesterolemia. Hyperlipidemia is usually chronic and requires ongoing medication to control blood lipid levels.
Scavenger receptors are a large and diverse superfamily of cell surface receptors. Its properties were first recorded in 1970 by Drs. Brown and Goldstein, with the defining property being the ability to bind and remove modified low density lipoproteins (LDL). Today scavenger receptors are known to be involved in a wide range of processes, such as: homeostasis, apoptosis, inflammatory diseases and pathogen clearance. Scavenger receptors are mainly found on myeloid cells and other cells that bind to numerous ligands, primarily endogenous and modified host-molecules together with pathogen-associated molecular patterns(PAMPs), and remove them. The Kupffer cells in the liver are particularly rich in scavenger receptors, includes SR-A I, SR-A II, and MARCO.

The low-density lipoprotein receptor (LDL-R) is a mosaic protein of 839 amino acids that mediates the endocytosis of cholesterol-rich low-density lipoprotein (LDL). It is a cell-surface receptor that recognizes apolipoprotein B100 (ApoB100), which is embedded in the outer phospholipid layer of very low-density lipoprotein (VLDL), their remnants—i.e. intermediate-density lipoprotein (IDL), and LDL particles. The receptor also recognizes apolipoprotein E (ApoE) which is found in chylomicron remnants and IDL. In humans, the LDL receptor protein is encoded by the LDLR gene on chromosome 19. It belongs to the low density lipoprotein receptor gene family. It is most significantly expressed in bronchial epithelial cells and adrenal gland and cortex tissue.

Apolipoprotein E (Apo-E) is a protein involved in the metabolism of fats in the body of mammals. A subtype is implicated in Alzheimer's disease and cardiovascular diseases. It is encoded in humans by the gene APOE.

Apolipoprotein B (ApoB) is a protein that in humans is encoded by the APOB gene. It is commonly used to detect risk of atherosclerotic cardiovascular disease.

The very-low-density-lipoprotein receptor (VLDLR) is a transmembrane lipoprotein receptor of the low-density-lipoprotein (LDL) receptor family. VLDLR shows considerable homology with the members of this lineage. Discovered in 1992 by T. Yamamoto, VLDLR is widely distributed throughout the tissues of the body, including the heart, skeletal muscle, adipose tissue, and the brain, but is absent from the liver. This receptor has an important role in cholesterol uptake, metabolism of apolipoprotein E-containing triacylglycerol-rich lipoproteins, and neuronal migration in the developing brain. In humans, VLDLR is encoded by the VLDLR gene. Mutations of this gene may lead to a variety of symptoms and diseases, which include type I lissencephaly, cerebellar hypoplasia, and atherosclerosis.

Familial hypercholesterolemia (FH) is a genetic disorder characterized by high cholesterol levels, specifically very high levels of low-density lipoprotein cholesterol, in the blood and early cardiovascular diseases. The most common mutations diminish the number of functional LDL receptors in the liver or produce abnormal LDL receptors that never go to the cell surface to function properly. Since the underlying body biochemistry is slightly different in individuals with FH, their high cholesterol levels are less responsive to the kinds of cholesterol control methods which are usually more effective in people without FH. Nevertheless, treatment is usually effective.

Lipoprotein(a) is a low-density lipoprotein variant containing a protein called apolipoprotein(a). Genetic and epidemiological studies have identified lipoprotein(a) as a risk factor for atherosclerosis and related diseases, such as coronary heart disease and stroke.

Apolipoprotein AI(Apo-AI) is a protein that in humans is encoded by the APOA1 gene. As the major component of HDL particles, it has a specific role in lipid metabolism.

Hepatic lipase (HL), also called hepatic triglyceride lipase (HTGL) or LIPC (for "lipase, hepatic"), is a form of lipase, catalyzing the hydrolysis of triacylglyceride. Hepatic lipase is coded by chromosome 15 and its gene is also often referred to as HTGL or LIPC. Hepatic lipase is expressed mainly in liver cells, known as hepatocytes, and endothelial cells of the liver. The hepatic lipase can either remain attached to the liver or can unbind from the liver endothelial cells and is free to enter the body's circulation system. When bound on the endothelial cells of the liver, it is often found bound to heparan sulfate proteoglycans (HSPG), keeping HL inactive and unable to bind to HDL (high-density lipoprotein) or IDL (intermediate-density lipoprotein). When it is free in the bloodstream, however, it is found associated with HDL to maintain it inactive. This is because the triacylglycerides in HDL serve as a substrate, but the lipoprotein contains proteins around the triacylglycerides that can prevent the triacylglycerides from being broken down by HL.
Endothelial lipase (LIPG) is a form of lipase secreted by vascular endothelial cells in tissues with high metabolic rates and vascularization, such as the liver, lung, kidney, and thyroid gland. The LIPG enzyme is a vital component to many biological processes. These processes include lipoprotein metabolism, cytokine expression, and lipid composition in cells. Unlike the lipases that hydrolyze Triglycerides, endothelial lipase primarily hydrolyzes phospholipids. Due to the hydrolysis specificity, endothelial lipase contributes to multiple vital systems within the body. On the contrary to the beneficial roles that LIPG plays within the body, endothelial lipase is thought to play a potential role in cancer and inflammation. Knowledge obtained in vitro and in vivo suggest the relations to these conditions, but human interaction knowledge lacks due to the recent discovery of endothelial lipase. Endothelial lipase was first characterized in 1999. The two independent research groups which are notable for this discovery cloned the endothelial lipase gene and identified the novel lipase secreted from endothelial cells. The anti-Atherosclerosis opportunity through alleviating plaque blockage and prospective ability to raise High-density lipoprotein (HDL) have gained endothelial lipase recognition.
Blood lipids are lipids in the blood, either free or bound to other molecules. They are mostly transported in a phospholipid capsule, and the type of protein embedded in this outer shell determines the fate of the particle and its influence on metabolism. Examples of these lipids include cholesterol and triglycerides. The concentration of blood lipids depends on intake and excretion from the intestine, and uptake and secretion from cells. Hyperlipidemia is the presence of elevated or abnormal levels of lipids and/or lipoproteins in the blood, and is a major risk factor for cardiovascular disease.

Apolipoprotein A-V is a protein that in humans is encoded by the APOA5 gene on chromosome 11. It is significantly expressed in liver. The protein encoded by this gene is an apolipoprotein and an important determinant of plasma triglyceride levels, a major risk factor for coronary artery disease. It is a component of several lipoprotein fractions including VLDL, HDL, chylomicrons. It is believed that apoA-V affects lipoprotein metabolism by interacting with LDL-R gene family receptors. Considering its association with lipoprotein levels, APOA5 is implicated in metabolic syndrome. The APOA5 gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.

Low density lipoprotein receptor-related protein 1 (LRP1), also known as alpha-2-macroglobulin receptor (A2MR), apolipoprotein E receptor (APOER) or cluster of differentiation 91 (CD91), is a protein forming a receptor found in the plasma membrane of cells involved in receptor-mediated endocytosis. In humans, the LRP1 protein is encoded by the LRP1 gene. LRP1 is also a key signalling protein and, thus, involved in various biological processes, such as lipoprotein metabolism and cell motility, and diseases, such as neurodegenerative diseases, atherosclerosis, and cancer.

Proprotein convertase subtilisin/kexin type 9 (PCSK9) is an enzyme encoded by the PCSK9 gene in humans on chromosome 1. It is the 9th member of the proprotein convertase family of proteins that activate other proteins. Similar genes (orthologs) are found across many species. As with many proteins, PCSK9 is inactive when first synthesized, because a section of peptide chains blocks their activity; proprotein convertases remove that section to activate the enzyme. The PCSK9 gene also contains one of 27 loci associated with increased risk of coronary artery disease.