High-density lipoprotein (HDL) is one of the five major groups of lipoproteins. Lipoproteins are complex particles composed of multiple proteins which transport all fat molecules (lipids) around the body within the water outside cells. They are typically composed of 80–100 proteins per particle and transporting up to hundreds of fat molecules per particle.

Low-density lipoprotein (LDL) is one of the five major groups of lipoprotein that transport all fat molecules around the body in extracellular water. These groups, from least dense to most dense, are chylomicrons, very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL) and high-density lipoprotein (HDL). LDL delivers fat molecules to cells. LDL is involved in atherosclerosis, a process in which it is oxidized within the walls of arteries.

A lipoprotein is a biochemical assembly whose primary function is to transport hydrophobic lipid molecules in water, as in blood plasma or other extracellular fluids. They consist of a triglyceride and cholesterol center, surrounded by a phospholipid outer shell, with the hydrophilic portions oriented outward toward the surrounding water and lipophilic portions oriented inward toward the lipid center. A special kind of protein, called apolipoprotein, is embedded in the outer shell, both stabilising the complex and giving it a functional identity that determines its role.
Very-low-density lipoprotein (VLDL), density relative to extracellular water, is a type of lipoprotein made by the liver. VLDL is one of the five major groups of lipoproteins that enable fats and cholesterol to move within the water-based solution of the bloodstream. VLDL is assembled in the liver from triglycerides, cholesterol, and apolipoproteins. VLDL is converted in the bloodstream to low-density lipoprotein (LDL) and intermediate-density lipoprotein (IDL). VLDL particles have a diameter of 30–80 nm. VLDL transports endogenous products, whereas chylomicrons transport exogenous (dietary) products. In the early 2010s both the lipid composition and protein composition of this lipoprotein were characterised in great detail.

Chylomicrons, also known as ultra low-density lipoproteins (ULDL), are lipoprotein particles that consist of triglycerides (85–92%), phospholipids (6–12%), cholesterol (1–3%), and proteins (1–2%). They transport dietary lipids from the intestines to other locations in the body. ULDLs are one of the five major groups of lipoproteins that enable fats and cholesterol to move within the water-based solution of the bloodstream. A protein specific to chylomicrons is ApoB48.
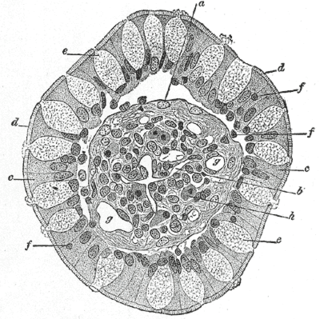
A lacteal is a lymphatic capillary that absorbs dietary fats in the villi of the small intestine.

Lipoprotein lipase (LPL) (EC 3.1.1.34, systematic name triacylglycerol acylhydrolase (lipoprotein-dependent)) is a member of the lipase gene family, which includes pancreatic lipase, hepatic lipase, and endothelial lipase. It is a water-soluble enzyme that hydrolyzes triglycerides in lipoproteins, such as those found in chylomicrons and very low-density lipoproteins (VLDL), into two free fatty acids and one monoacylglycerol molecule:

Apolipoproteins are proteins that bind lipids to form lipoproteins. They transport lipids in blood, cerebrospinal fluid and lymph.

The low-density lipoprotein receptor (LDL-R) is a mosaic protein of 839 amino acids that mediates the endocytosis of cholesterol-rich low-density lipoprotein (LDL). It is a cell-surface receptor that recognizes apolipoprotein B100 (ApoB100), which is embedded in the outer phospholipid layer of very low-density lipoprotein (VLDL), their remnants—i.e. intermediate-density lipoprotein (IDL), and LDL particles. The receptor also recognizes apolipoprotein E (ApoE) which is found in chylomicron remnants and IDL. In humans, the LDL receptor protein is encoded by the LDLR gene on chromosome 19. It belongs to the low density lipoprotein receptor gene family. It is most significantly expressed in bronchial epithelial cells and adrenal gland and cortex tissue.

Apolipoprotein E (Apo-E) is a protein involved in the metabolism of fats in the body of mammals. A subtype is implicated in Alzheimer's disease and cardiovascular disease. It is encoded in the human by the gene APOE.

Apolipoprotein B (ApoB) is a protein that in humans is encoded by the APOB gene.
Vitellogenin is a precursor of egg yolk that transports protein and some lipid from the liver through the blood to the growing oocytes where it becomes part of the yolk. Normally, it is only found in the blood or hemolymph of females, and can therefore be used as a biomarker in vertebrates of exposure to environmental estrogens which stimulate elevated levels in males as well as females. "Vitellogenin" is a synonymous term for the gene and the expressed protein. The protein product is classified as a glycolipoprotein, having properties of a sugar, fat and protein. It belongs to a family of several lipid transport proteins.

Apolipoprotein AI(ApoA-I) is a protein that in humans is encoded by the APOA1 gene. As the major component of HDL particles, it has a specific role in lipid metabolism.

Apolipoprotein C-III also known as apo-CIII, and apolipoprotein C3, is a protein that in humans is encoded by the APOC3 gene. Apo-CIII is secreted by the liver as well as the small intestine, and is found on triglyceride-rich lipoproteins such as chylomicrons, very low density lipoprotein (VLDL), and remnant cholesterol.

Apolipoprotein C-IV, also known as apolipoprotein C4, is a protein that in humans is encoded by the APOC4 gene.
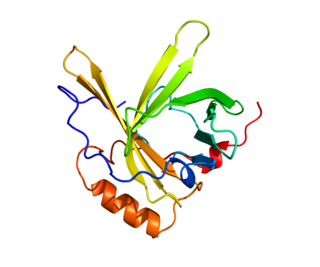
Apolipoprotein D(ApoD) is a protein that in humans is encoded by the APOD gene. Unlike other lipoproteins, which are mainly produced in the liver, apolipoprotein D is mainly produced in the brain and testes. It is a 29 kDa glycoprotein discovered in 1963 as a component of the high-density lipoprotein (HDL) fraction of human plasma. It is the major component of human mammary cyst fluid. The human gene encoding it was cloned in 1986 and the deduced protein sequence revealed that ApoD is a member of the lipocalin family, small hydrophobic molecule transporters. ApoD is 169 amino acids long, including a secretion peptide signal of 20 amino acids. It contains two glycosylation sites (aspargines 45 and 78) and the molecular weight of the mature protein varies from 20 to 32 kDa (see figure 1).
Blood lipids are lipids in the blood, either free or bound to other molecules. They are mostly transported in a protein capsule, and the density of the lipids and type of protein determines the fate of the particle and its influence on metabolism. The concentration of blood lipids depends on intake and excretion from the intestine, and uptake and secretion from cells. Hyperlipidemia is the presence of elevated or abnormal levels of lipids and/or lipoproteins in the blood, and is a major risk factor for cardiovascular disease.

Apolipoprotein A-IV is plasma protein that is the product of the human gene APOA4.
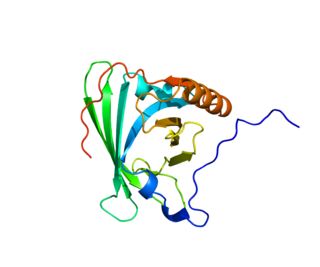
Apolipoprotein M is a protein that in humans is encoded by the APOM gene.

Apolipoprotein F is a protein that in humans is encoded for by the APOF gene.















